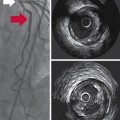
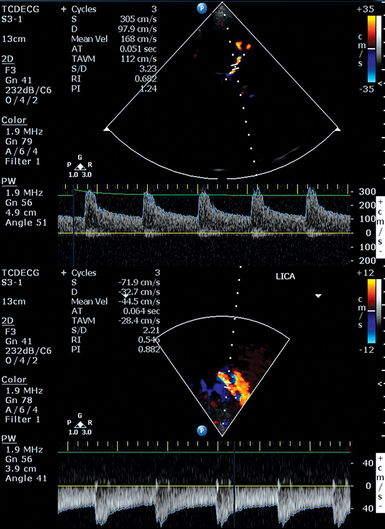
3 The management of aneurysmal subarachnoid hemorrhage (aSAH) and its accompanying sequelae integrates transcranial Doppler (TCD) ultrasonography for the surveillance of the emergence and course of vasospasm (VS) and delayed cerebral ischemia.1 TCD surveillance of patients after aSAH has been advocated by the two most recent aSAH treatment guidelines, and practice standards for its use are published.2–4 This disease is complex to manage, and TCD has emerged as an inexpensive, noninvasive tool for monitoring intracerebral hemodynamic changes seen with SAH as well as for evaluating other neurologic conditions, including intracranial arterial stenosis, arteriovenous malformations, emboli of different origin, venous sinus thrombosis, brain death, ischemic stroke and clot lysis, and sickle cell disease.5,6 The phenomenon of the diminution of blood flow transiting through the cerebral vasculature seen after aSAH or severe neurotrauma resulting from the decrease in the caliber of arteries is referred to as VS.7,8 Arterial spasm after aSAH was originally described by Ecker in 1951 and has since been the subject of decades of laboratory research and clinical investigations. The phenomena of “angiographic VS” and “clinical VS” are often discussed, both of which may culminate in eventual delayed cerebral ischemia (DCI) and permanent neurologic deficits. The exact cause of VS is not clearly understood.1,7 VS often occurs most intensely adjacent to the subarachnoid clot burden but can occur distant from the majority of the subarachnoid blood and is predicted by clot volume, age, location, and density of the SAH seen on the initial computed tomography (CT) scan.9,10 Because of more aggressive early surgical or endovascular treatment of ruptured aneurysms, fatality from early re-rupture has now been replaced by complications of hydrocephalus and VS as the most common and serious causes of morbidity and mortality after aSAH.11,12 Thus monitoring for VS and DCI with noninvasive techniques, such as TCD, remains a critical tool in the management of this disease.1 Angiographic VS is common after aSAH, occurring in as many as 67% of patients, with the highest incidence occurring between days 10 and 17 after aSAH.12 Classically, VS is reported to occur from day 4 to 14, but variations on this general rule are common.1,3,4,7,13 The incidence of early angiographic VS, detected within 48 hours of aneurysm rupture, occurs in 10% to 13% of aSAH patients and is associated with prior aSAH, large aneurysms, intraventricular hemorrhage, and worsened 3-month outcome and morbidity.14 It is now well recognized that VS is also seen after traumatic SAH.8 VS is a clinical diagnosis, and findings range from nonfocal neurologic signs, such as confusion, increasing somnolence, and combativeness, to focal and localizable neurologic deficits from stroke. In 1982, Aaslid provided the first descriptions of the use of TCD for early detection of VS.15 The gold standard for the diagnosis of cerebral VS has remained digital subtraction angiography (DSA), although this modality is not practical for use as a frequent monitor for VS. Computed tomography angiography (CTA) has emerged as a potentially helpful tool in the evaluation of VS and is often used along with TCD.3,4 Because of its portability for bedside testing, noninvasive nature, ease of repeated testing, and lack of known adverse side effects from its use, TCD has become one of the most used screening tools for monitoring patients with aSAH for the development of VS in practice currently. In common practice, TCD is performed daily to twice daily from the first day after presenting with aSAH until VS subsides. TCD is an operator-dependent examination, although up to 8% of patients may have skull insonation windows too thick to allow evaluation of cerebral blood flow velocities (CBFVs) in cm/sec.13 As described by Aaslid, TCD uses the underlying principle that the velocity of blood flow in a conduit is inversely related to the diameter of that conduit, and that as diameter is decreased, velocity will increase, and vice versa. An indirect evaluation of the vessel diameter is achieved by using the Doppler effect upon red blood cells by calculating the Doppler shift between the frequencies of the transmitted and received ultrasound waves.16 TCD provides several indices that assist in the clinical decision making for patients with aSAH. The CBFV is the most used metric and is also described by parameter of the mean CBFV (mCBFV), the peak systolic flow velocity (Vs), and the end diastolic flow velocity (Vd). In clinical practice, the mCBFV is typically reported, but the other parameters are used to calculate the resistance index (RI) and the pulsatility index (PI). Both RI and PI are presumptive measures of downstream vascular resistance and may predict intracranial compliance or intracranial pressure.17,18 Clinical decision-making based on this relationship of the PI currently remains controversial.19 Elevated RI and PI may also occur secondary to distal VS or intracranial stenosis. The derivation of RI and PI are shown in Figure 3-1. The Lindegaard (ratio) index (LI) is a method of correcting for increases in hyperdynamic systemic flow velocities that are either physiologically or medically induced in patients with aSAH (e.g., because of triple-H therapy: hypervolemia, hypertension, hemodilution). To calculate the LI, the mCBFV of the middle cerebral artery (MCA) is compared with an ipsilateral extracranial vessel, which is usually the proximal internal carotid artery (ICA) (Figure 3-2). This ratio helps to distinguish global hyperemia from VS, particularly when the LI is greater than 6.3,20 Others have suggested that an intracranial to extracranial ratio for the basilar artery (BA), for instance, comparison of the BA mCBFV to the extracranial vertebral artery (VA) mCBFV, of greater than 3 is highly sensitive and specific for posterior circulation VS.21 The extracranial/intracranial mCBFV ratio can be especially helpful in aSAH in the setting of the global hyperemia that accompanies the often-used triple-H therapy. Figure 3-2 Moderate vasospasm in the left middle cerebral artery after aneurysmal subarachnoid hemorrhage as detected by real-time transcranial Doppler (TCD): mean flow velocity in the left middle cerebral artery is greater than130 cm/sec (top), and the LI calculated by the ratio of the latter to the mean flow velocity detected in the ipsilateral proximal internal carotid artery (LICA, bottom) is greater than 3. (Courtesy Dr. D. Karakitsos.) TCD measurements alone may not correlate with angiographic or symptomatic VS. Differing hemodynamic states may alter the effects of decreased vessel lumen diameter on flow resistance as measured by TCD. It is postulated that with moderate VS, cerebral autoregulation compensates for perfusion pressure reduction in the region of spasm, if arterial blood pressure is above the lower limit of autoregulation. The flow velocity then increases as lumen area falls, yielding good correlation between angiographic and TCD measured spasm.22 As stenosis increases, the volume of flow is reduced, and velocity remains highly independent of diameter from ineffective attempts at cerebral autoregulation. When blood pressure is augmented with triple-H therapy, flow increases and ischemic symptoms may improve; mean flow velocity (MFV) may paradoxically have higher values than when normotensive. In this scenario, the correlation between TCD velocity and the degree of angiographic VS is likely to be poor. If vessel diameter worsens further, lower mCBFV and reduction of flow to critical values may manifest with ischemia.22 It is recommended that TCD studies be performed in the intensive care unit (ICU) for patients with aSAH.3,4 This data can be interpreted based on absolute criteria for VS or used to see trends in the tempo of VS over the course of several days.3 Some have proposed a point scoring system for VS, with reported sensitivity and specificity of both at 96%.23 The correlation between TCD mCBFV with decreases in vessel diameter on angiography have been most convincing for examinations of the MCA.1 Ideal placement of the reference standard for mCBFV and extracranial/intracranial mCBFV ratios is debated, although potential cutoff values where mild, moderate, and severe VS is likely present is presented in Table 3-1.
Transcranial doppler in aneurysmal subarachnoid hemorrhage
(CONSULTANT LEVEL EXAMINATION)
Overview
Vasospasm after aneurysmal subarachnoid hemorrhage
Transcranial doppler as a monitor for cerebral vasospasm
Indices and technical aspects of transcranial doppler ultrasonography
Interpretation of data from transcranial doppler
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Radiology Key
Fastest Radiology Insight Engine