Transient ischemic attack (TIA) can convey a high imminent risk for the development of a major stroke and is therefore considered to be a medical emergency. Recent evidence indicates that TIA with imaging proof of brain infarction represents an extremely unstable condition with early risk of stroke that is as much as 20 times higher than the risk after TIA without tissue damage. The use of neuroimaging in TIA is therefore critical not only for diagnosis but also for accurate risk stratification. In this article, recent advances in diagnostic imaging, categorizations, and risk stratification in TIA are discussed.
Each year, approximately 200,000 to 500,000 patients are diagnosed by a physician as having experienced a transient ischemic attack (TIA) in the United States. An additional 300,000 to 700,000 individuals experience neurologic symptoms suggestive of a TIA but never seek medical attention for their symptoms. The clinical syndrome of TIA designates that the abnormality in the cardiovascular system leading to compromised blood flow to the brain is unstable and, if not properly treated, may also cause a debilitating ischemic stroke. The most remarkable characteristic of TIA is, perhaps, the temporal information it conveys that relates to the timing of an upcoming stroke. The excess risk after TIA can be imminent; the risk is highest within hours of a TIA and declines steadily within the ensuing days, weeks, and months ; nearly half of strokes occurring within the next 30 days occur within the first 24 hours after a TIA. It is estimated that 12% to 30% of patients report a history of TIA soon before their stroke and approximately a quarter of them occur during the hours before the stroke. TIA constitutes a true medical emergency. Early initiation of preventive treatment of TIA (for instance within 24 hours instead of 20 days) can reduce the 90-day risk of stroke by approximately 80%. Although rapid and accurate diagnosis and urgent initiation of treatment are key to the management of TIA, given that nearly half of the population reports a brief episode of focal loss of brain function (either TIA or TIA mimics) at one point in their lifetime, indiscriminate use of diagnostic and therapeutic resources may exhaust the health care system. It is critical to accurately identify patients who are most likely to benefit from further diagnostic investigations and rapid treatment. The purpose of this review is to provide an overview on the traditional TIA concept, introduce the new tissue-based definition, and discuss the potential utility of advanced diagnostic imaging and risk stratification in TIA.
Clinical diagnosis of TIA
According to the World Health Organization criteria proposed in 1988, TIA is defined as rapidly developed clinical signs of focal or global disturbance of cerebral function, lasting less than 24 hours, with no apparent nonvascular cause. The National Institute of Neurologic Disorders and Stroke Report published in 1990 defines TIA as brief episodes of focal loss of brain function for less than 24 hours, thought to be caused by ischemia, which can usually be localized to that portion of the brain supplied by one vascular system. Both of these traditional definitions are based on the assumption that rapid resolution of symptoms in TIA indicates an ischemic insult that is transient at the tissue level. Traditional definitions also assume that clinical judgment as to whether the pattern of signs and symptoms fit into a specific arterial territory is an accurate means of attributing transient symptoms to ischemia. Recent advances in neuroimaging have substantially changed our understanding of TIA. At present, we know that none of these assumptions are quite correct. TIA is not necessarily transient at the tissue level; approximately one-third of traditionally defined TIAs are associated with permanent ischemic tissue injury. Likewise, focal symptoms localizable to an arterial territory do not a priori indicate an ischemic mechanism; several nonischemic mechanisms, including seizures, subdural hemorrhage, intracerebral hemorrhage, brain tumors, multiple sclerosis, and migraine, can cause transient neurologic symptoms confined to a vascular territory.
Transient events characterized by symptoms that are focal but not clearly attributable to a known cause are considered atypical or uncertain TIA. Clinical features that are not considered to be typical of an ischemic attack include gradual buildup of symptoms (more than 5 minutes), spread of symptoms from one body part to another (without passing the midline), progression of symptoms from one type to another, isolated disturbance of vision in both eyes characterized by the occurrence of positive phenomena, isolated sensory symptoms with remarkably focal distribution, isolated brain stem symptoms, and the occurrence of identical spells over a period longer than 1 year. Transient focal atypical spells are common, corresponding to approximately 1 of every 5 emergency room visits because of transient neurologic symptoms. Approximately 10% of patients with atypical symptom characteristics reveal an acute brain lesion on diffusion-weighted magnetic resonance imaging (DWI), suggesting that atypical spells do not exclusively represent a nonischemic subset and may, therefore, not be as benign as previously considered [Hakan Ay, MD, unpublished Massachusetts General Hospital data, 2011].
The opportunity for objective assessment of clinical deficit in TIA is very limited. Approximately 60% of TIAs last for less than 1 hour, and two-thirds of those lasting for less than 1 hour last for less than 10 minutes. Only less than 10% of patients can be examined by physicians when they are fully symptomatic. The inevitable use of historical information contributes to the variability in TIA diagnosis; reported rates of disagreement in the diagnosis of TIA by history vary between 42% and 86% among neurologists. This variability in TIA diagnosis obviously hampers the clinical and research utility of the term TIA. Recent advances in neuroimaging have revolutionized the evaluation of TIA and provided an opportunity to overcome many of the shortcomings of the clinical-based approach by introducing an objective component to its definition. The following section summarizes the current state of knowledge on the utility of neuroimaging in attributing a transient spell to brain ischemia.
Brain imaging findings in TIA
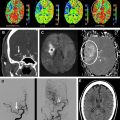
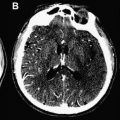
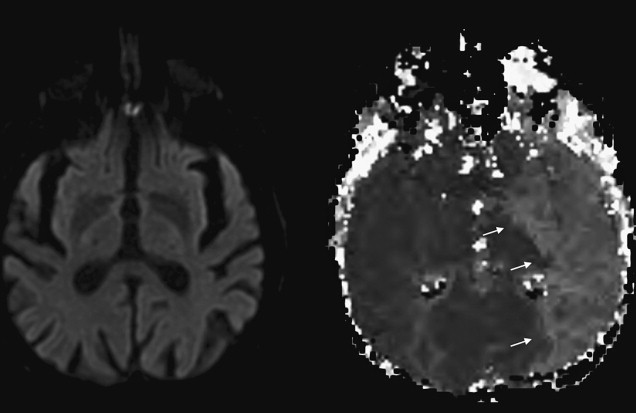
The introduction of first computed tomography (CT) and later magnetic resonance imaging (MRI) to the evaluation of TIA have challenged the conventional view by demonstrating that clinically transient events are not necessarily transient at the tissue level. Approximately one-third of patients with the clinical syndrome of TIA develop a clinically relevant brain infarct. The first report of imaging proof of brain infarct in patients with TIA dates back to 1979. Perrone and coworkers reported small hypodense areas on CT consistent with infarction in 12 of the 35 patients with TIA. Four years later, in 1983, Waxman and Toole coined the term “cerebral infarction with transient deficit” to describe transient episodes associated with CT evidence of brain infarction in a clinically relevant location. Since then several researchers have used CT to observe patients with TIA and reported variable rates of brain infarcts ranging from 4% to 34%. Subsequent studies with MRI have revealed somewhat higher rates of infarcts changing between 21% and 67%. Although infarct rates on CT and MRI are similar, the diagnostic yield of these two imaging methods in identifying the clinically relevant or clinically appropriate infarct is quite different. The ability to distinguish acute infarcts from chronic lesions is critical to be able to tie a brain infarct to the transient clinical event. CT has limited sensitivity in identifying clinically related infarcts because infarcts observed in TIA are often very small, lack edema and mass effect, and demonstrate no or very subtle contrast enhancement. In a study of 149 patients with TIA, only 16% of infarcts suspected to be acute based on CT assessment had lesion characteristics consistent with acute infarcts detected on DWI. In another study of 57 patients with TIA, lesions designated as clinically appropriate and acute on conventional MRI (Fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images) overlapped with an acute lesion detected by DWI in only 48% of the patients, indicating that the so-called appropriate lesion on conventional MRI was not acute and therefore not related to the transient clinical event in more than half of the patients ( Fig. 1 ). DWI’s ability to differentiate between acute and chronic infarcts is clearly a strength in conditions associated with small infarcts such as TIA. Another strength of DWI is its high signal to background contrast. Using DWI, acute small infarcts can be visualized that are not visible on CT or conventional MRI in approximately one-third of patients with TIA. Current TIA guidelines recommend DWI as the preferred method of imaging in patients with TIA. Nevertheless, MRI cannot be tolerated or is contraindicated in approximately 10% of patients, and this limits its widespread applicability. In addition, availability, feasibility, and affordability of MRI for use in emergency management of TIA are limited in many practices. According to a survey based on emergency department visits in the United States between 1992 and 2001, MRI was the first line of imaging in only less than 5% of patients with TIA. A more recent survey conducted in 2008 indicates that 15% of Canadian neurologists routinely use MRI in their practice for managing TIA. Despite a trend toward more frequent use of MRI in recent years, most patients with TIA are currently underevaluated. Training of first-line physicians, formation of hospital systems that enable rapid access to MRI, and development of newer imaging techniques with higher spatial resolution and lower cost are critical to enhance the diagnostic evaluation of TIA.
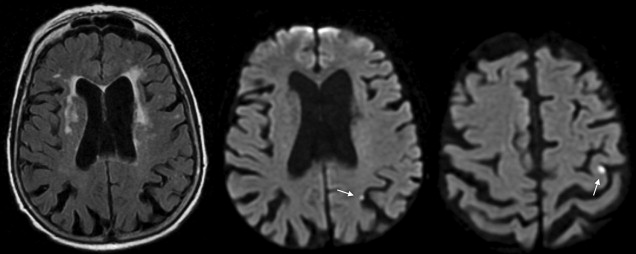
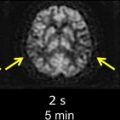
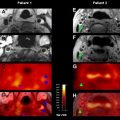
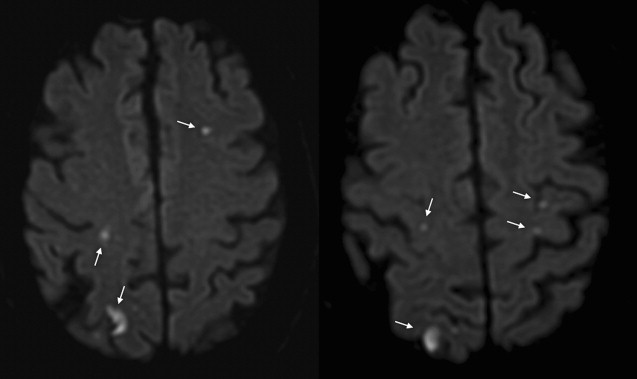
The most important characteristic of TIA-related infarcts on DWI is their strikingly small size (see Fig. 1; Figs. 2 and 3 ). Infarcts as small as 0.07 mL can occur during a TIA. About 96% of all infarcts in TIA are smaller than 1 mL. The authors have previously coined the term “footprints of transient ischemia” to describe such punctate lesions on DWI that remain after complete resolution of TIA symptoms. TIA-related infarcts can occur in any part of the brain including clinically important structures such as the brain stem, internal capsule, and motor cortex, as well as less important or silent brain regions. The probability of infarct on DWI increases as the symptom duration increases, yet this relationship is not consistent across all studies. The authors have observed brain infarcts occurring in patients with symptoms lasting for as short as 30 seconds as well as normal DWI despite symptoms lasting for several hours. Such evidence suggests that the pathophysiology of TIA includes not only tissue damage but also a component of recovery; it suggests the possibility of interplay between several factors such as the size of the ischemic insult and the robustness of the affected neuronal circuitry, including perhaps the strength of the axonal connections as well as of the underlying neurovascular substrate. There are clearly several fruitful areas for further investigation into how the recovery from documentable tissue damage that is clearly present in patients with TIA might be able to be extended to larger stroke insults.
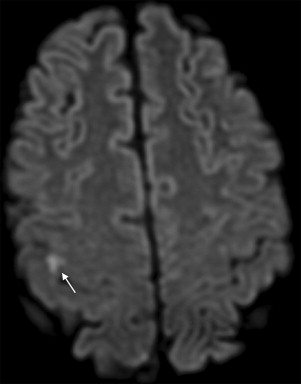
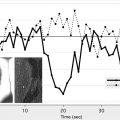
Although the presence of infarct on DWI indicates that the mechanism of transient clinical event is ischemic in origin, the opposite is not always true; DWI results can be negative when in fact transient symptoms are caused by ischemia. DWI has limited sensitivity for very small infarcts, particularly in the brain stem location. In addition, a short lasting episode of ischemia that is not severe enough to cause permanent tissue injury may cause symptoms in the absence of lesions detected by DWI. The combined use of DWI and perfusion-weighted MRI may improve the sensitivity for detection of tissue ischemia ( Fig. 4 ). Based on observational case studies, perfusion-weighted MRI seems to provide evidence consistent with ischemia in an additional 3% to 16% of patients with TIA on top of DWI. The diagnostic utility of perfusion-weighted MRI remains to be confirmed in unbiased large datasets. Limited spatial resolution of currently available perfusion-weighted MRI techniques is also of concern. Improvements in perfusion techniques in the future may overcome these concerns by enhancing the reliability of diagnoses for punctate regions of ischemia that typically occur in TIA.
Brain imaging findings in TIA
The introduction of first computed tomography (CT) and later magnetic resonance imaging (MRI) to the evaluation of TIA have challenged the conventional view by demonstrating that clinically transient events are not necessarily transient at the tissue level. Approximately one-third of patients with the clinical syndrome of TIA develop a clinically relevant brain infarct. The first report of imaging proof of brain infarct in patients with TIA dates back to 1979. Perrone and coworkers reported small hypodense areas on CT consistent with infarction in 12 of the 35 patients with TIA. Four years later, in 1983, Waxman and Toole coined the term “cerebral infarction with transient deficit” to describe transient episodes associated with CT evidence of brain infarction in a clinically relevant location. Since then several researchers have used CT to observe patients with TIA and reported variable rates of brain infarcts ranging from 4% to 34%. Subsequent studies with MRI have revealed somewhat higher rates of infarcts changing between 21% and 67%. Although infarct rates on CT and MRI are similar, the diagnostic yield of these two imaging methods in identifying the clinically relevant or clinically appropriate infarct is quite different. The ability to distinguish acute infarcts from chronic lesions is critical to be able to tie a brain infarct to the transient clinical event. CT has limited sensitivity in identifying clinically related infarcts because infarcts observed in TIA are often very small, lack edema and mass effect, and demonstrate no or very subtle contrast enhancement. In a study of 149 patients with TIA, only 16% of infarcts suspected to be acute based on CT assessment had lesion characteristics consistent with acute infarcts detected on DWI. In another study of 57 patients with TIA, lesions designated as clinically appropriate and acute on conventional MRI (Fluid-attenuated inversion recovery (FLAIR)- and T2-weighted images) overlapped with an acute lesion detected by DWI in only 48% of the patients, indicating that the so-called appropriate lesion on conventional MRI was not acute and therefore not related to the transient clinical event in more than half of the patients ( Fig. 1 ). DWI’s ability to differentiate between acute and chronic infarcts is clearly a strength in conditions associated with small infarcts such as TIA. Another strength of DWI is its high signal to background contrast. Using DWI, acute small infarcts can be visualized that are not visible on CT or conventional MRI in approximately one-third of patients with TIA. Current TIA guidelines recommend DWI as the preferred method of imaging in patients with TIA. Nevertheless, MRI cannot be tolerated or is contraindicated in approximately 10% of patients, and this limits its widespread applicability. In addition, availability, feasibility, and affordability of MRI for use in emergency management of TIA are limited in many practices. According to a survey based on emergency department visits in the United States between 1992 and 2001, MRI was the first line of imaging in only less than 5% of patients with TIA. A more recent survey conducted in 2008 indicates that 15% of Canadian neurologists routinely use MRI in their practice for managing TIA. Despite a trend toward more frequent use of MRI in recent years, most patients with TIA are currently underevaluated. Training of first-line physicians, formation of hospital systems that enable rapid access to MRI, and development of newer imaging techniques with higher spatial resolution and lower cost are critical to enhance the diagnostic evaluation of TIA.
The most important characteristic of TIA-related infarcts on DWI is their strikingly small size (see Fig. 1; Figs. 2 and 3 ). Infarcts as small as 0.07 mL can occur during a TIA. About 96% of all infarcts in TIA are smaller than 1 mL. The authors have previously coined the term “footprints of transient ischemia” to describe such punctate lesions on DWI that remain after complete resolution of TIA symptoms. TIA-related infarcts can occur in any part of the brain including clinically important structures such as the brain stem, internal capsule, and motor cortex, as well as less important or silent brain regions. The probability of infarct on DWI increases as the symptom duration increases, yet this relationship is not consistent across all studies. The authors have observed brain infarcts occurring in patients with symptoms lasting for as short as 30 seconds as well as normal DWI despite symptoms lasting for several hours. Such evidence suggests that the pathophysiology of TIA includes not only tissue damage but also a component of recovery; it suggests the possibility of interplay between several factors such as the size of the ischemic insult and the robustness of the affected neuronal circuitry, including perhaps the strength of the axonal connections as well as of the underlying neurovascular substrate. There are clearly several fruitful areas for further investigation into how the recovery from documentable tissue damage that is clearly present in patients with TIA might be able to be extended to larger stroke insults.