Treatment-Related Changes
17.1 The Postoperative Thorax
17.1.1 Partial Lung Resection
Surgical Techniques
Partial lung resection is performed for therapeutic and, less commonly, also for diagnostic purposes:
Diagnostic resection: This is mostly done for histologic analysis of suspicious pulmonary nodules or for diagnosis of diffuse parenchymal lung diseases. In general, less lung tissue is removed for diagnostic purposes compared with therapeutic interventions. The technique used is usually wedge resection where a wedge-shaped piece of lung tissue measuring a few centimeters is removed with a stapler. Such methods which are not based on anatomic structures are known as atypical resections (▶Table 17.1).
Therapeutic resection: This is usually done for surgical treatment of lung tumors and, less commonly, also for elimination of chronic inflammatory processes. It normally involves anatomic resection except in the case of metastasis surgery (see following sections): an anatomically defined part of the lung is removed, often a lung lobe (lobectomy), in contrast to the atypical resection described above. In the right lung, treatment sometimes requires the removal of two lobes: either the upper and middle lobes (upper bilobectomy) or the middle and lower lobes (lower bilobectomy). Removal of the right upper and lower lobes as bilobectomy is not technically feasible. Pneumonectomy is needed instead.
The maximum extension of the resection is determined by the size and location of the pathologic process and is limited by the expected postoperative lung function. Occasionally, the standard oncologic procedure, i.e., at least lobectomy, is not a feasible option for patients with a limited preoperative lung function. Therefore, in such cases, surgery may be confined to removal of individual lung segments (segmental resection).
Lung metastases are usually removed by means of atypical resection. Unlike in lung carcinoma, anatomic resection is generally not needed, thus ensuring that as much lung parenchyma as possible is preserved. The standard surgical procedures are wedge resection and laser resection. In the latter, a laser is used to extract the metastatic lesion including a safety margin in a virtually circular manner from the surrounding lung tissue, and the pleura is sutured to close the surgical defect.
Table 17.1 Surgical techniques in lung resection | ||||
|---|---|---|---|---|
|
Radiologic Findings
Anatomic Resection
Radiologic findings in anatomic resection:
Hemithorax volume loss:
High-riding diaphragm.
Mediastinal shift to the operated side.
Lobar fissures not identifiable.
Pleural thickening.
Pleural cavity (generally only in the immediate postoperative phase).
Pulmonary consolidations (in particular in segmental resection).
Middle lobe atelectasis (in right upper lobe resection).
Hilar enlargement (in the immediate postoperative phase).
The most salient finding on chest radiography in anatomic resection is the volume loss of the operated lung: the diaphragm on the operated side is somewhat higher than normal and the mediastinum has shifted slightly to the operated side (▶Fig. 17.1). There is often remarkably little noticeable volume loss in lobectomies and segmental resections because the remaining lung parenchyma completely fills the space in the chest cavity and contains comparatively more air than the contralateral side. Occasionally, apart from nonrecognition of one lobar fissure, there are virtually no signs of the previous lobectomy on chest radiography.
 Fig. 17.1 Right lower lobe resection. Radiograph. Right highriding diaphragm and slight mediastinal shift to the right. |
If the remaining lung parenchyma is unable to completely fill the chest cavity, an air-containing pleural cavity is seen postoperatively. This is usually replaced within a few days by a pleural effusion that over time is converted to connective tissue, thus giving rise to a permanent pleural thickening (▶Fig. 17.2). Rarely, the air-containing cavity persists for months or even years.
 Fig. 17.2 Pleural thickening (arrows) after right lower lobe resection. Furthermore, persistent small, right-apical pneumothorax (arrowhead). Radiograph. |
 Fig. 17.3 Pulmonary consolidation following resection of the posterior right upper lobe segment (arrows). Radiographs. (a) Posteroanterior image. (b) Lateral image. |
Whereas lobectomies and bilobectomies usually leave no radiologically discernible scars in the lung parenchyma, segmental resections are often associated with radiologic findings. Only entire lung lobes, but not segments, are separated by the visceral pleura. During lobectomy, the entire lobe can be detached virtually atraumatically from the surrounding lung parenchyma along this boundary. In contrast, segmental resection requires surgical dissection in the lung parenchyma causing identifiable consolidations in the operated lobe at the site of the resected segment (▶Fig. 17.3).
Surgical treatment of lung carcinoma routinely includes systematic hilar and mediastinal lymphadenectomy. Occasionally, hilar enlargement is seen on the operated side in the immediate postoperative course (▶Fig. 17.4); this resolves within a few days.
At times, a small triangular opacity based at the apex of the diaphragm can be observed following upper lobe resection. This “juxtaphrenic peak” is caused by cranial displacement of an accessory fissure or of septa associated with the pulmonary ligament. On postoperative CT, surgical changes to the bronchial system can be identified additionally. Broncho- and angioplastic surgical techniques are needed to treat centrally located tumors or large hilar lymph node metastases. Conversancy with these techniques is useful for correct interpretation of the imaging findings. Parts of the central bronchi or vessels are resected in these procedures (known as sleeve resections) and a distal part of the bronchus or artery is anastomosed with the mainstem bronchus or artery, respectively For example, in right upper lobe bronchial sleeve resection, the distal mainstem bronchus, including the origin of the upper lobe bronchus as well as the proximal intermediate bronchus, is resected, and then the distal intermediate bronchus is connected to the proximal mainstem bronchus (▶Fig. 17.5).
Atypical Resection
Wedge resections leave virtually linear consolidations in the lung parenchyma, which originate from the pleural lung surface and are usually a few centimeters long (▶Fig. 17.6). The metal clips applied with the stapler may be visible.
Laser resections leave rounded defects in the lung parenchyma. Since the visceral pleura is closed again with a suture over the resection defect, this often leads to development of pleural-based rounded, cyst-like structures. Their relatively thin wall is caused by the laser-induced coagulation necrosis of the lung parenchyma (▶Fig. 17.7). On chest radiography, such surgical defects manifest as cavities (▶Fig. 17.8), resembling lung abscesses. These normal postoperative findings after laser resection must not be misinterpreted as an infectious complication.
 Fig. 17.4 Postoperative hilar enlargement following right upper lobe resection. Magnified section of a radiograph. Postoperative right-sided chest tubes as well as right perihilar clip chain. |
Wedge and laser resections are frequently combined in metastasis surgery. Hence, the sequelae of both procedures may manifest simultaneously.
Complications
General complications common for all surgical techniques are described in Chapter 17.1.8. The following are specific to partial lung resections:
Right upper lobe resections present a risk of postoperative hypoaeration of the middle lobe which now occupies the position of the resected upper lobe in the chest cavity. This causes upward displacement of the middle lobe bronchus, which normally arises in an anteroinferior direction from the intermediate
bronchus, and can now become kinked at the site of origin. The resultant bronchial stenosis causes early postoperative middle lobe atelectasis (▶Fig. 17.9). If this condition persists, middle lobe syndrome characterized by chronic recurrent middle lobe infections can develop; in the worst case, eventual middle lobe resection is required.
bronchus, and can now become kinked at the site of origin. The resultant bronchial stenosis causes early postoperative middle lobe atelectasis (▶Fig. 17.9). If this condition persists, middle lobe syndrome characterized by chronic recurrent middle lobe infections can develop; in the worst case, eventual middle lobe resection is required.
 Fig. 17.6 Bilateral wedge resections. CT image. Linear, pleuralbased consolidations in both upper lobes. |
 Fig. 17.7 Laser resection in right upper lobe. CT images. (a) Preoperative finding: metastasis in the right upper lobe (arrow). (b) Postoperative day 10: thick-walled cavity. (c) Third postoperative month. (d) Ninth postoperative month. |
17.1.2 Pneumonectomy
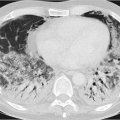
At times, resection of an entire lung, known as pneumonectomy, may be necessary to treat central lung carcinomas. The chest cavity is empty apart from air immediately postoperative and completely fills with fluid over the course of several weeks. Later the fluid becomes organized and transforms to connective tissue, the so-called fibroserothorax.1 Imaging demonstrates increased radiolucency of the pneumonectomy cavity in the immediate postoperative course. The air-fluid level seen on upright chest radiography continues to rise upward over time until the entire hemithorax exhibits opacities of soft-tissue density (▶Fig. 17.10).
 Fig. 17.9 Middle lobe atelectasis following right upper lobe resection (arrows). CT image. Postoperative right-sided chest tube. |
Occasionally, a smoothly marginated, paramediastinal opacity is seen in early chest radiographs lateral to the bronchial stump (▶Fig. 17.11).2 This is consistent with a muscle or pericardial flap attached intraoperatively to the mainstem bronchus stump to prevent bronchial stump fistula.
In addition to general complications, pneumonectomy is associated with a number of specific complications now described below.1
Pneumonectomy causes sudden-onset hyperperfusion of the remaining lung, pulmonary arterial pressure increase, and right heart overload. Postoperative pulmonary edema is seen in up to 5% of cases. In milder cases, this demonstrates the radiographic findings of hydrostatic pulmonary edema, while in severe cases it resembles adult respiratory distress syndrome. The underlying mechanism of this deeply feared and usually fatal complication is not fully understood. It is thought to be linked to elevated hydrostatic intravascular pressure and increased capillary permeability.1
 Fig. 17.10 Status post right pneumonectomy. Radiographs. (a) Supine image on postoperative day 1. (b) Upright image on postoperative day 3. (c) After 9 months. |
 Fig. 17.11 Status post right pneumonectomy. Radiograph. Muscle flap introduced into chest cavity for coverage of the bronchial stump (arrows). |
The sudden drop in fluid levels in the pneumonectomy cavity is suggestive of bronchial stump leakage, which is associated with a considerable mortality rate. Likewise, new onset of postoperative mediastinal or chest wall emphysema points to bronchial stump fistula (▶Fig. 17.12).
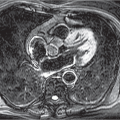
Postpneumonectomy syndrome typically occurs in younger patients during the first postoperative year, predominantly after right-sided pneumonectomy. Clinical symptoms include increasing exertional dyspnea, inspiratory stridor, and recurrent lung infections. The hyperexpanded lung leads to mediastinal displacement to the operated side, in turn causing ipsilateral tracheal displacement and hyperextension of the mainstem bronchus of the remaining lung (▶Fig. 17.13).
17.1.3 Surgery for Pleural Diseases
Surgery for pleural diseases is usually indicated in settings of pleural empyema, malignant tumors, or less commonly because of persistent pneumothorax.
Pleurodesis
Pleurodesis, where the pleural layers are stuck together, may be performed to treat a symptomatic malignant pleural effusion. To that effect, a chemical substance, e.g., talc, is introduced into the pleural space. The ensuing inflammatory reaction causes the parietal pleura and visceral pleura to stick together and thus prevent the development of a new large pleural effusion.
On postoperative chest radiography, there is normally marked regression of pleural effusion compared with that seen prior to pleurodesis. It is crucial to ensure the absence of pleural air collections since these cause separation of the pleural layers, preventing the desired adhesion of the parietal and visceral pleura.
Around postoperative day 3 to 5, onset of a concomitant reaction of the lung parenchyma lasting around 2 weeks is sometimes seen on imaging (▶Fig. 17.14).3 On chest radiography, progressive pulmonary opacities occur.4 CT demonstrates coarse linear opacities and intralobular lines, ground-glass opacities, and smaller consolidations in the peripheral lung parenchyma (▶Fig. 17.15). Most of these findings will have resolved within a few weeks.
Imaging shows persistent smooth or nodular pleural thickening. On PET-CT, these areas of pleural thickening can exhibit considerable FDG uptake, hampering differential diagnosis versus pleural tumor manifestations.5 The talc used in talc pleurodesis is often visualized on CT as deposits of calcific density in the pleural space, thus mimicking calcified pleural plaques.6
 Fig. 17.13 Postpneumonectomy syndrome following left pneumonectomy. (a) Radiograph. Tracheal displacement to the left and anterior herniation of the right lung into the left hemithorax (arrows). (b) CT image. Hyperextension of the right mainstem bronchus located anterior to the spine (arrow). |
Pleurectomy and Decortication
Pleurectomy, i.e., removal of the parietal pleura, is usually used for surgical treatment of pneumothorax. Lung decortication involves partial or complete removal of the visceral pleura from the lung, e.g., in pleural empyema or pleural mesothelioma. The main purpose of decortication is to remove the thickened pleura as this hampers expansion and aeration of the underlying lung.
On chest radiography and CT, it is not possible in settings of pleural enlargement to reliably distinguish between surgical sequelae, tumor, and inflammation. At times, considerable
atelectasis of the subpleural lung tissue is seen in the immediate postoperative phase, especially after decortication. These areas of atelectasis are surgical manifestations that usually resolve within a few days.
atelectasis of the subpleural lung tissue is seen in the immediate postoperative phase, especially after decortication. These areas of atelectasis are surgical manifestations that usually resolve within a few days.
17.1.4 Surgery for Pneumothorax
Surgical treatment of recurrent pneumothorax entails atypical resection of bullous changes in the lung parenchyma. In addition, partial pleurectomy can be performed to achieve adhesion of the pleural layers and thus prevent recurrence of pneumothorax.
The sequelae of atypical partial lung resection can be identified on imaging. This procedure is generally performed as wedge resection. Bullae are usually found at the apices of the upper and lower lobes.
The chief determinant of a successful treatment outcome is the immediate postoperative contact between the parietal and visceral pleura. Even the most minute pleural air inclusion is clinically relevant and must be meticulously sought on postoperative chest radiography. The information presented on partial lung resection and surgery for pleural diseases also applies for chest radiography after surgery for pneumothorax.
17.1.5 Lung Transplantation
Lung transplantation has become established as a treatment option for end-stage diffuse parenchymal lung diseases, chronic obstructive pulmonary disease, and pulmonary hypertension. Both single- and double-lung transplants are carried out, also in combination with heart transplantation. Imaging has an important role in follow-up of lung transplant patients for early detection of complications. These and the associated imaging findings are presented in ▶Table 17.2.
 Fig. 17.15 Pleurodesis reaction 2 weeks after right talc pleurodesis. CT image. Diffuse pleural thickening, subpleural consolidations, mild ground-glass opacities, and coarse intralobular lines. |
The most dangerous complication in the first posttransplantation year is bacterial infection; later the patient’s life is at risk because of chronic rejection.7
Opportunistic infections following organ transplantation are discussed in Section 5.3. Of particular relevance for lung transplant patients is cytomegalovirus infection. This can still manifest even months or years later, whereas the greatest risk by fungal infections is seen in the first posttransplantation weeks.
Special complications occur at different time points8:
Immediate complications (within the first 24 h): Donor-recipient size mismatch becomes evident during or immediately after transplant surgery. Malposition of the peri- or intraoperatively inserted catheters and tubes or complications related to their placement (e.g., tube malposition as well as pneumo- or hemothorax after insertion of a central venous line) also become clinically or radiologically manifest within the first postoperative hours. Preformed antibodies to donor-specific antigens (e.g., HLA or AB0 antigens) are thought to be responsible for fulminant hyperacute rejection manifesting immediately after transplantation and leading to death.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree