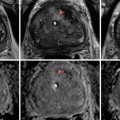
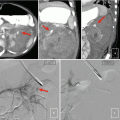
Fig. 16.1
(a) Surgical prostate specimen removed showing the characteristic gross features of BPH, including nodular growth within the transition zone (TZ) surrounding the urethra (U). The arrows point to additional areas within the transition zone which are enlarged and compress the peripheral zone (PZ) posteriorly against the prostatic capsule. (b) Histopathology of BPH showing glandulo-stromal hyperplasia
Lower urinary tract symptoms, or LUTS, is the accepted terminology used to describe the symptoms which are often associated with BPH and benign prostatic enlargement (BPE) [4, 5]. They reflect not only a direct (static) component of prostatic growth and obstruction but also a dynamic component of obstruction as well [6]. It is the static component of obstruction that results from an increase in number and volume of epithelial and smooth muscle cells, as well as connective tissue within the prostatic stroma. The dynamic component of obstruction results from an increase in the smooth muscle tone and resistance from the enlarging gland. Finally factoring into the LUTS perceived by the patient is the bladder response to obstruction, aging, and other less obvious influences [7]. This bladder component results in a spectrum of responses, varying from detrusor underactivity to detrusor overactivity.
LUTS can be broadly classified into one of three, often overlapping categories (Fig. 16.2) [8]. As mentioned above, none of the voiding symptoms under these categories are specific to BPH. In fact, cross-sectional studies performed in the United States and Europe have shown that the prevalence of LUTS in women is equivalent to that of men, suggesting that the pathophysiology of LUTS is much more complex than a simple obstructive process, as described above [9, 10]. However, under the right circumstances, in a male 45 years or older, in the absence of other potential non-BPH causes for LUTs, clinicians may safely attribute LUTs to BPH and BPE [6]. When confirmed by the presence of low flow and high voiding pressures on urodynamic studies, benign prostatic obstruction (BPO) is the most common diagnosis [4, 5, 8].
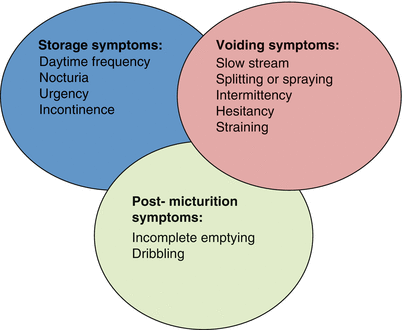

Fig. 16.2
Current classification system of lower urinary tract symptoms (LUTS) (From Abrams et al. [8], with permission)
Measuring the Severity of BPH
The AUA Symptom Index (AUA-SI) and the International Prostate Symptom Score (IPSS) are identical, interchangeable, validated instruments that have proven indispensable in grading both the severity of LUTS and their impact on an individual’s quality of life [11, 12]. This seven-question, self-administered questionnaire elicits a 1-month account of the patient’s symptoms that encompass storage (frequency, nocturia, urgency), voiding (force of stream, the need to strain to empty), and post-micturition (intermittency and sensation of incomplete voiding) symptoms. The overall score allows patients to be stratified into different categories of severity: mild (score 0–7), moderate (score 8–19), and severe (score 20–35). There is one additional quality of life (QoL) question that is designed to accompany the IPSS. It directly elicits the degree of bother associated with the severity symptom score.
Two additional objective parameters that are often used in the literature to describe the effects of a particular intervention are the maximal urinary flow rate (Qm) and the residual volume of urine detected in the urinary bladder after voiding (post-void residual, PVR). While urodynamics remains the standard for diagnosing BPO, these parameters are helpful in providing additional objective data about the voiding dysfunction. Of note, there is some temporal variation and lack of consistency on repeat testing in both of these parameters, which should never be used as the sole indicator of the value of an intervention.
The Burden of BPH and LUTS
Autopsy studies have demonstrated there is a gradual increase in the prevalence of BPH with each decade of life beyond age 30 years [13, 14]. This coincides with a generalized increase in the prevalence of BPE, based upon transrectal ultrasound [15] and MRI volumetric measurements [16]. Concomitant to these age-related increases in the prevalence of BPH and BPE, there is also a dramatic increase in the prevalence of LUTS [17, 18].
One of the most widely referenced observational studies is the Olmsted County Study, which demonstrated that moderate LUTs (AUA symptom score 8 or higher) were present in 26 % of men in their 50s and in close to 50 % of men in their 80s. The problem transcends race, as 40 % of African-American males have moderate LUTS, and transcends global boundaries, as well [10].
Medical therapy has proven highly successful and is often the first line of therapy for bothersome LUTS that are associated with BPH [19]. Data suggest that up to 40 % of patients presenting for treatment of BPH are prescribed one or more pharmaceuticals, including alpha adrenoreceptor antagonists, 5-alpha reductase inhibitors, and anticholinergic agents [20]. Unfortunately, up to 30 % of men will ultimately discontinue therapy, because of bothersome adverse effects or lack of perceived benefit [21, 22].
While the utilization of medical therapy for BPH has increased over the last three decades, surgery continues to be used to treat symptoms resulting from this disorder. Data from the Medicare Carrier File [23] reveal that BPH procedures actually increased 44 % from 88,868 in 1999 to 127,786 in 2005, primarily due to an increase in the use of minimally invasive treatment options, such as lasers and thermotherapy (TUNA and microwave treatment). Interestingly, a recent update of this series showed that between 2005 and 2008, there was a slight decline in BPH surgery which is not easily explained and which affected almost all of the surgical modalities (Fig. 16.3). Nevertheless, the surgical treatment of BPH remains a very important part of the management of this disease.
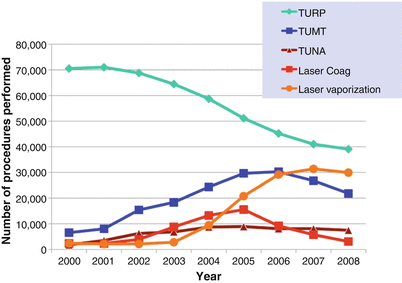

Fig. 16.3
Trends in the surgical treatment of BPH since the start of the millennium (From Malaeb et al. [23], with permission)
Indications for Surgical Treatment of BPH
Surgical intervention is an appropriate treatment alternative for well-informed patients with moderate (IPSS score 9–19) or severe LUTs (IPSS score 20–35) and for patients who have developed acute urinary retention or other BPH-related complications [6, 24]. Many of these patients have symptoms that are unresponsive to medical therapy or are no longer desirous of long-term pharmacotherapy. BPH-related complications include chronic urinary retention, recurrent urinary infections resulting from BPO, recalcitrant hematuria, and renal impairment secondary to obstruction.
Surgical Options
Transurethral Resection of the Prostate (TURP)
Still recognized as the gold standard for surgical management of LUTS secondary to BPO in prostates < 80 ml, transurethral resection of the prostate (TURP) [6, 24] continues to evolve, despite almost 100 years in the urologic armamentarium. While a complete description of the technique is beyond the scope of this chapter, the basic premise of the TURP is to endoscopically resect all obstructing tissue within the transition zone, from the bladder neck to the verumontanum. This is a visible landmark on the floor of the prostate that demarcates the boundary of the striated sphincter and is where the ejaculatory ducts exit the gland.
While TURP represents a significant improvement over open prostatectomy in terms of recovery, it is far from perfect. In the perioperative period, adverse events associated with TURP (see Table 16.1) include a higher than acceptable risk of perioperative blood transfusions, TUR syndrome, perioperative urinary infections, delayed bleeding resulting in readmission, reoperation, and placement of a catheter [25–31]. Additional shortcomings of TURP are that the patient usually requires inpatient observation in the perioperative period, as well as requiring an indwelling catheter for a period of time following surgery. Between 6 and 15 % of patients undergoing TURP will require additional surgery for adenomatous regrowth, urethral strictures, or bladder neck contractures at 8–10 years [32, 33], and others will have permanent retrograde ejaculation or erectile dysfunction.
Table 16.1
Perioperative complications associated with TURP from select large series 1989–2011
Study | # pts. | Mortality (%) | Transfused (%) | TURP syndrome | Urinary infection (%) |
|---|---|---|---|---|---|
Mebust et al. (1989) [25] | 3885 | 0.23 | 6.4 | 2.0 | 2.3 |
Doll et al. (1992) [26] | 388 | 2.8 | 22 | NS | 14 |
Horninger et al. (1996) [27] | 1211 | 0 | 7.6 | 2.8 | 2.4 |
Uchida et al. [28] | |||||
1971–1985 | 1930 | 0.2 | 20.2 | 0.5 | 2.5 |
1985–1996 | 1931 | 0.5 | 6.1 | 0.3 | 0.6 |
Berger et al. (2004) [29] | 271 | 0 | 2.6 | 1.1 | NS |
Reich et al. (2008) [30] | 9197 | 0.1 | 2.9 | 1.4 | 3.6 |
Mamoulakis et al. (2011) [31] | 198 | 0.5 | 4 | 0.5 | 4.5 |
Over the last two decades, a number of improvements in instrumentation have resulted in a reduction of the perioperative morbidity associated with TURP, as can be seen in Table 16.1. These include dramatic improvements in fiber-optic technology and the utilization of increasingly high-definition camera units and monitors. By magnifying and enhancing the visibility of the operative field, these modifications have improved the precision of the surgical resection and have directly impacted the surgeon’s ability to maintain adequate hemostasis. As a result, the transfusion risk associated with TURP has declined from nearly 20 % in the 1970s and 1980s to below 5 % in most contemporary series [28, 30–34].
The irrigant used for the standard TURP is usually glycine, which allows for a monopolar, high-frequency electroresection of prostatic tissue, with the ability to also use coagulation currents for hemostasis. Because glycine has an osmolarity of 200 mOsm/L, it does not cause hemolysis when it is systemically absorbed through the prostate during resections, as does sterile water. However, absorption of glycine may cause dilutional hyponatremia, particularly with prolonged resection times, during deep resections in which venous sinuses are exposed and when the height of the irrigant is more than 3 feet above the patient. This dilutional hyponatremia is the underlying mechanism for TUR syndrome, which may initially elicit visual changes, dizziness, headaches, nausea, and vomiting. As TUR syndrome progresses, it may precipitate apnea, seizures, and a variety of circulatory abnormalities (hypertension, hypotension, bradycardia, and arrhythmias). If left untreated, the patient may develop life-threatening pulmonary and cerebral edema, which must be addressed with diuresis. Clinical experience in treating this disorder is essential, as too rapid correction of the hyponatremia may elicit central pontine myelinolysis.
The incorporation of continuous flow irrigation systems in most contemporary resectoscope units is one measure that may or may not assist in prevention of TUR syndrome. The use of this system, which fosters continuous inflow and outflow of irrigant through separate channels within the resectoscope sheath, allows bladder pressures to remain relatively constant during the course of resection. By not having to disassemble the resectoscope numerous times during the procedure to empty the bladder of blood-tinged irrigation, it expedites the procedure, subsequently reducing the amount of blood loss and the amount of irrigation that the patient absorbs. There are no definitive studies that have proven that continuous flow resectoscopes are effective at preventing TUR syndrome, but most urologic surgeons prefer them in order to perform the resection in an uninterrupted fashion.
While contemporary instrumentation associated with monopolar TURP (M-TURP) has helped reduce the risk of perioperative complications, they have not entirely eradicated them. In fact, patients with larger prostates and those who present with preoperative indwelling catheters for urinary retention continue to pose special challenges for M-TURP. Generally, these situations require longer resection times and are associated with a higher risk of intraoperative blood loss [29, 30]. Other patients, such as those who have indwelling catheters, as well as those that need to continue anticoagulants in the perioperative period represent additional high-risk patients.
Bipolar-TURP and Bipolar Vaporization of the Prostate (BPVP)
The development of bipolar transurethral resection and vaporization procedures represents a very significant advance in the evolution of TURP. In traditional monopolar surgical units, electrical current travels through the patient’s body to reach a grounding pad that is placed somewhere on the surface of the patient. Electrical current is readily diffused by ions within irrigation fluid. As a result, monopolar TURP (M-TURP) must be carried out in water, glycine, sorbitol, or mannitol. Bipolar circuitry is comprised of an active and a return pole attached to a single support on the resectoscope itself [34]. The electrical current that is generated with these units is not attenuated by ions within the irrigation solution. As a result, these units are quite functional in saline.
There are currently four bipolar resection devices that are available: the transurethral resection in saline (TURis) system (Olympus), the plasmakinetic system (Gyrus-PK), the Karl Storz bipolar device, and the Richard Wolf bipolar device. Each of these systems has the capability to resect or vaporize tissue, as well as perform coagulation functions.
Technique
Irrespective of whether the surgeon is using a bipolar loop electrode for a B-TURP or the button (Olympus) or half-moon (Karl Storz) electrodes for bipolar vaporization of the prostate (BPVP), the technical maneuvers, thought process, and overall strategic approach are very similar to the performance of an M-TURP (see Figs. 16.4, 16.5, and 16.6). This results in a relatively short learning curve for most urologic surgeons who are proficient in M-TURP.
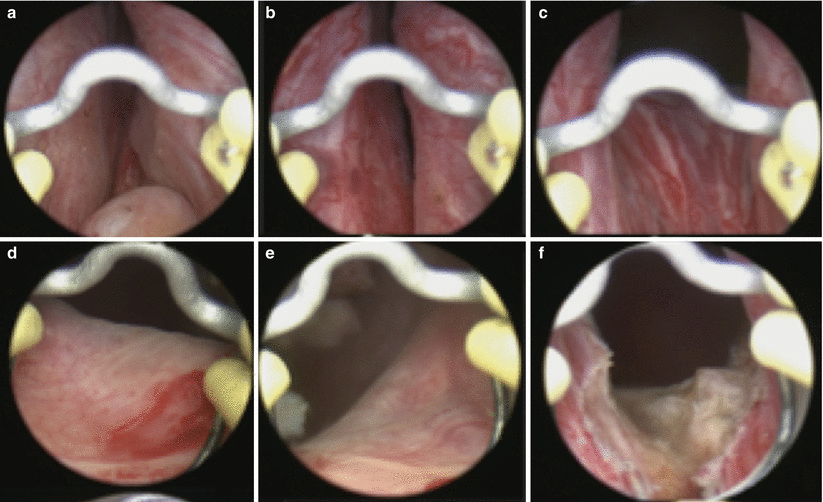
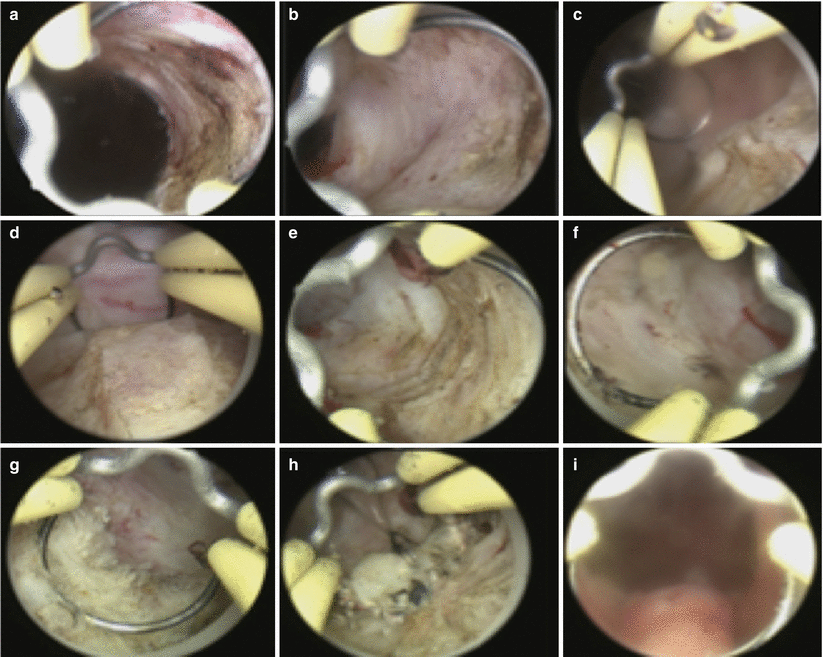
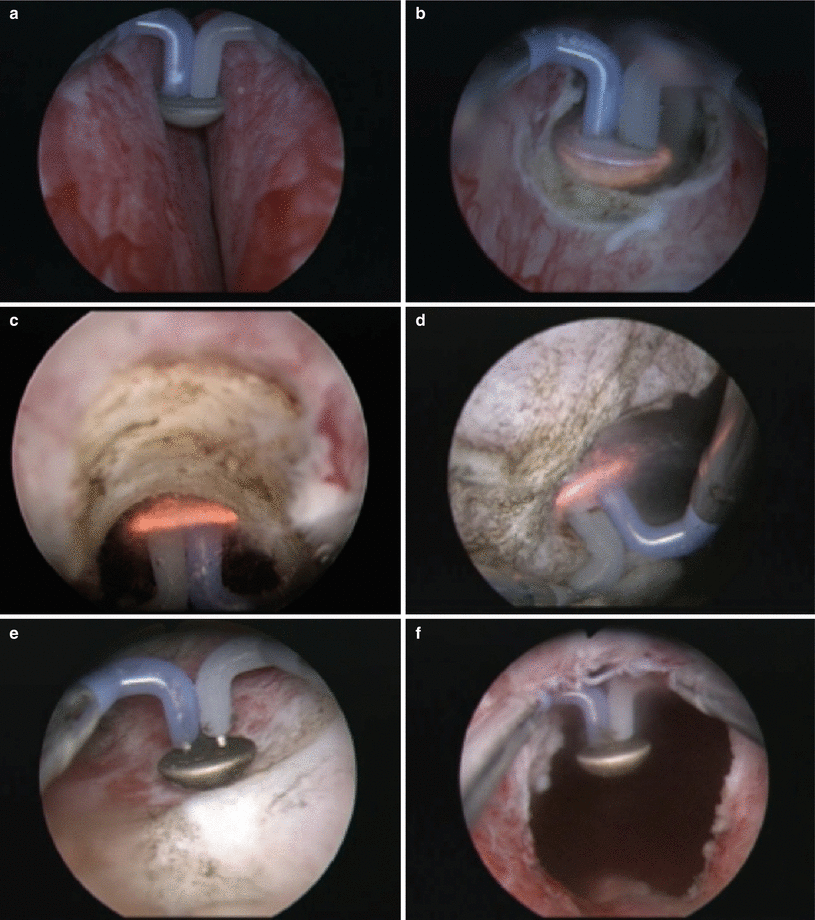

Fig. 16.4
Individual steps for B-TURP. In the upper panel, the prostate is visualized from the (a) verumontanum, (b) mid prostate, and (c) bladder neck. In the second row, the (d) right ureteral orifice and (e) left ureteral orifice are inspected prior to (f) resecting a portion of the floor to allow for chip mobilization and continuous fluid irrigation

Fig. 16.5
B-TURP. The resection begins at the bladder neck at the 1 o’clock position (a). Adenoma is resected until the circular fibers of the surgical capsule can be seen (b). The resection then moves clockwise from 2 to 5 o’clock positions (c–e). The next step is to resect the right lateral lobe from the 11 o’clock position to the 7 o’clock position (f, g). The floor is then resected, and residual apical tissue is removed last (h). At the termination of the procedure, the prostatic capsule can be visualized from the verumontanum as completely devoid of residual adenoma (i)

Fig. 16.6
Bipolar plasma vaporization (BPVP) of the prostate using the “button”-vapo-resection electrode (Olympus, Hamburg, Germany). During the procedure, the electrode is moved back and forth as it gently contacts the underlying prostatic tissue. As can be seen in panel d, a cloud of vaporization occurs as the tissue virtually disintegrates. The procedure offers excellent visualization, coagulation is used for addressing more prominent blood vessels, and the surgeon can readily identify the prostatic capsule (panel e) before moving on to another area (a) Bilateral prostate before initiating vaporization. (b) Vaporization at bladder neck. (c) Vaporization at 12 o’clock posterior anterior. (e) Vaporization of the floor of prostate with visible capsular fibers. (f) Bladder (black) visualized from the verumontanum at the completion of the procedure (From Geavlete et al. [35], with permission)
Results
A recent meta-analysis identified 33 independent randomized clinical trials (RCTs) comparing B-TURP to M-TURP [36]. While RCTs suffer from relatively low patient numbers, are predominantly single-center series, provide poor long-term follow-up, and lack patient and investigator blinding to the various procedures, B-TURP was found to have several advantages over M-TURP. First, B-TURP was associated with a significant reduction in the decline of perioperative serum Hb levels, which translated to a reduction in the need for blood transfusions. Of over 2800 evaluable patients from RCTs, the transfusion risk associated with B-TURP was half of that associated with M-TURP (2.2 % versus 4.4 %, p = 0.0009). Additionally, the risk of TUR syndrome in 1339 patients undergoing M-TURP was 1.4 %, versus 0 of 1329 patients undergoing B-TURP, p = 0.002. While the data supported a reduction in the length of stay and the duration of catheterization, these did not reach meaningful clinical relevance. The risk of clot retention and immediate return to the operating room was less following B-TURP, and with short-term follow up, there was no difference in the incidence of urethral strictures, bladder neck contractures, or urinary infections.
In terms of efficacy at 1 year, there was no difference between B-TURP and M-TURP in terms of IPSS, QoL score, PVR, or prostate volume. There was a slightly higher maximal flow rate (Qm) associated with B-TURP, but this was not clinically meaningful.
Tables 16.2 and 16.3 present complications and intermediate-level efficacy from five RCTs with follow-up between 18 and 48 months [35, 37–41]. This supports that B-TURP is comparable, in terms of efficacy as M-TURP. It also is associated with a lower risk of perioperative complications.
Table 16.2
RCTs with intermediate follow-up, comparing complications of bipolar TURP and monopolar TURP
Study | # pts. | F/U (Mos) | Volume (g) | Resection time (mins) | Transfused (%) | TUR syndrome (%) | Urethral strictures (%) | BN contracture (%) | Reoperation (%) for BPH |
|---|---|---|---|---|---|---|---|---|---|
Autorino et al.(2009) [37] | 32 B, PK-TURP | 48 | 51.6 | 53 | NS | NS | 1.9 | 1.9 | 1.9 |
31 M-TURP | 47.5 | 49 | 4.0 | 2.0 | 2.0 | ||||
Chen et al.(2010) [38] | 50 B-TURP | 24 | 60.2 | 59 | 2 | 0 | 4 | 2 | NS |
50 M-TURP | 59.1 | 60 | 6 | 0 | 6 | 4 | NS | ||
Geavlete et al.(2011) [39] | 170 BPVP | 18 | 54.1 | 39* | 1.2 | 0 | 4.7 | 0.6 | 3.5 |
170 B-TURP | 53.7 | 52.1 | 1.8 | 0 | 6.5 | 3.5 | 9.4 | ||
170 M-TURP | 54.8 | 55.6 | 6.5* | 1.8 | 5.3 | 4.1 | 8.8 | ||
141 B-TURP | 36 | 63.8 | 39.5 | 6.4 | 0 | 8.2 | 6.6 | 18.9 | |
138 M-TURP | 63.5 | 39.1 | 2.9 | 0.7 | 9.3 | 1.9 | 14.8 | ||
Komura et al.(2014) [41] | 63 B-TURP | 36 | 51 | 79.5* | 1.6 | 0 | NS | NS | 0 |
62 M-TURP | 53 | 68.4 | 7 | 0 | NS | NS | 2 |
Table 16.3
Efficacy in RCTs with mid-level follow-up comparing monopolar TURP with bipolar procedures
Study | System (s) | # patients | Follow-up mos.) | Pre-/post-opIPSS (% change) | Pre-/post-opQm (% change) | Pre-/post-opPVR (% change) |
|---|---|---|---|---|---|---|
Autorino et al.(2008) [37] | PK-TURP | 32 B, PK-TURP 31 M-TURP | 48 | 24.2/6.4 (73) 24.3/6.9 (71) | 7.1/19.8 (179) 6.2/21.2 (241) | 80/42 (48) 75/45 (40) |
Chen et al.(2010) [38] | TURis | 50 B-TURP 50 M-TURP | 24 | 22.8/3.7 (84) 21.8/3.8 (83) | 7.1/25.5 (259) 7.9 /24.8 (214) | NS NS |
Geavlete et al.(2011) [39] | Button-BPVP & TURis | 170 BPVP 170 B-TURP 170 M-TURP | 18 | 24.2/5.0 (79) 24/7.9 (67) 24.2/8.3 (66) | 6.6/23.7 (259) 6.2/20.6 (232) 6.4/20.2 (215) | 91/29 (68) 96/31 (67) 88/33 (63) |
AUTOCON II 400 | 122 B-TURP 107 M-TURP | 24–36 | 23.4/8 (66) 23.1/7 (70) | 8.8/19.5 (122) 8.8/19.4 (120) | 91.8/27.8 (70) 100.5/20.2 (80) | |
Komura et al.(2014) [41] | TURis | 63 B-TURP 62 M-TURP | 36 | 23.7/5.2 (78) 22/4.2 (81) | 6.4/16.8 (163) 7.1/18.6 (161) | 43.7/10.3 (76) 47.4/6.6 (86) |
Laser Treatment of BPH
Lasers have increasingly been incorporated into the urological armamentarium for BPH surgery over the last 25 years. An array of laser systems have been developed, which by virtue of their intrinsic properties may produce a variety of effects on BPH tissue [42, 43]. These include desiccation, coagulation, and vaporization. Lasers may be used in a variety of capacities; they can be used for tissue ablation, incision, resection, and enucleation.
A fundamental working knowledge of laser physics is necessary in order to understand how and why particular lasers were chosen for management of BPH. As we will see, certain lasers are not well suited for treatment of BPH.
Basic Laser Physics
There are 92 different elements, each with a single nucleus and a variable number of electrons, which are constantly in motion. The addition of energy to a system of atoms causes excitation of the electrons, which absorb the energy and are raised into a higher state of energy. When the electron returns to its ground state, the energy emitted by the atom is released as a photon of light. The wavelength of the photon that is released is distinct and characteristic to the particular element.
Figure 16.7 demonstrates a very simple laser system. Energy is delivered to the atoms within the lasing medium by an external source. In this case it is the flash tube, which then excites the atoms. The excited atoms collide with other excited atoms, exponentially increasing the amount of energy within the laser chamber.
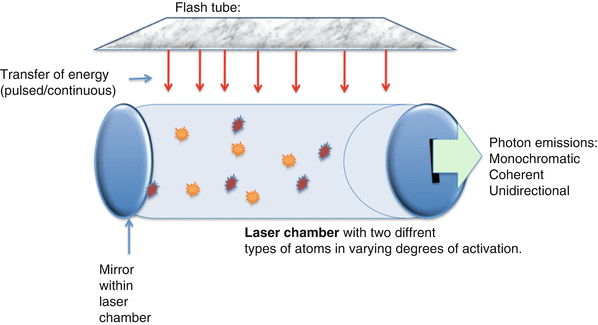

Fig. 16.7
Characteristics of a simple laser system. The flash tube delivers energy to the laser chamber either in a continuous or pulsatile fashion. This causes excitation of the atoms within the laser chamber which ricochet against each other and against the mirror at the end of the laser chamber. As energized atoms return to the ground state, the photons released are reflected within the chamber. Some will be released through the small slit-like opening in the right side of the chamber as laser light
The photons of light released within the lasing medium are reflected in the laser chamber by a series of mirrors that are positioned at the ends of the laser chamber. One of these mirrors contains a small slit which allows some of the photons to escape. The escaping energy has all of the properties that are characteristics of light emitted by a laser: it is monochromatic, coherent, and unidirectional.
When a laser is directed into a medium, energy is absorbed and dissipated along the trajectory of entry. In the case of a well-vascularized, soft tissue medium such as the prostate, the absorbed laser energy is converted into heat, which may increase the temperature along the trajectory of the site of entry. The temperatures may reach 70–90 °C, at which time, desiccation and coagulation of the tissue occur. At higher temperatures, above 100 °C, tissue vaporization occurs.
Figure 16.8 indicates the specific wavelengths of a number of laser systems currently used to treat BPH. Superimposed on this graph are the absorption coefficients for hemoglobin water and melatonin, which ultimately dictate the effects of the particular laser on the tissue. This absorption coefficient indicates at what depth most of energy is absorbed by the tissue. Finally, the depth of penetration determines the linear depth that the laser into the tissue before it is completely absorbed.
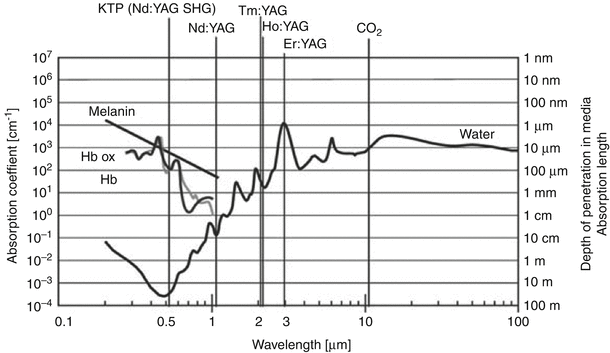

Fig. 16.8
Characteristics of various lasers used in the management of BPH (From Teichmann et al. [43], with permission)
Lasers Used for BPH
There are a number of different types of lasers that have been utilized in the management of BPH. It should be noted that this remains a work in progress. Improvements in the types of lasers, wavelength of energy used, optical delivery systems, precision of laser applications, and cost reduction continue to improve laser technology and extend its potential applications [44].
The Nd:YAG laser was one of the first laser systems used for treatment of BPH. From Fig. 16.8 one can see that this type of laser is well absorbed by hemoglobin, resulting in excellent hemostasis and coagulation. Because of the high penetration coefficient, however, most of the tissue effects resulting from treatment with the Nd:YAG laser occur deep within the prostate and not at the surface. As a result, patients who were subjected to BPH treatment with the Nd:YAG laser would often complain of pain, burning, and inflammatory symptoms for weeks, often requiring a catheter to be until the surface layers sloughed. As a result of these early experiences, the visual laser ablation of the prostate (VLAP) and transurethral ultrasound-guided laser-induced prostatectomy (TULIP), both using Nd:YAG laser technology, are no longer in use.
By adding a KTP crystal into the laser resonator, the KTP Nd:YAG laser emits energy with a wavelength of 532 nm, which is within the green spectrum of light. At this wavelength, the KTP Nd:YAG laser is strongly absorbed by hemoglobin, which is abundant in a well-vascularized tissue such as the prostate. With a short absorption length, there are rapid increases in tissue temperature at the point of entry into the tissue. This promotes surface vaporization with a seam of coagulation necrosis at the base [42].
Holmium (Ho):YAG lasers have also become very popular laser systems for treatment of BPH. Similar to the Nd:YAG, the crystalline matrix remains yttrium, aluminum, and garnet. Neodymium doping is replaced by a combination of chromium, thulium, and holmium. Chromium is excited easily by white light from a flash lamp. It then transfers energy to the thulium ions in the ground state, which doubles the number of excited ions with half the initial excitation energy. This facilitates energy transfer to the holmium ions, activating the laser, which then emits a wavelength of 2140 nm. The absorption length of the holmium laser is short due to strong absorption by water molecules, resulting in tissue effects that extend <0.5 mm into the tissue. This results in rapid onset of tissue vaporization with each laser pulse (Fig. 16.9).
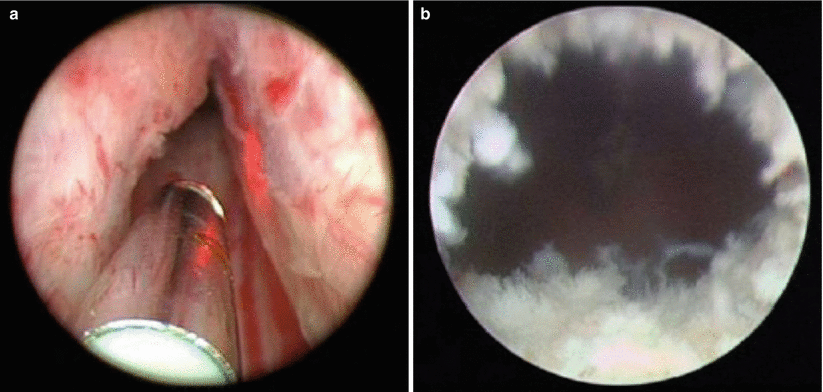

Fig. 16.9
Endoscopic view of holmium laser ablation of the prostate (HoLAP), demonstrating the post-treatment defect which appears blanched and irregular. (a) Endoscopic view of holmium laser of the prostate HoLAP, before initiating the procedure. (b) Demonstrating the post treatment defect which appears blanched and irregular
From a practical standpoint, the holmium laser has been found to be extremely versatile. It is currently being used in both ablative and enucleation procedures. In the latter capacity, the holmium laser can create a pulsatile vaporization bubble, which separates the prostatic adenoma from the surgical capsule and creates a rim of coagulation necrosis underneath [45].
The Thulium:YAG laser (not shown in Fig. 16.9) emits at a wavelength of 2013 nm, which is very similar to the Ho:YAG. This accounts for its similar interactions within tissue. What makes the Thulium:YAG laser different is that the output is delivered as a continuous wave rather than in a pulsatile manner. This allows for faster and more efficient tissue vaporization [45].
Contemporary Laser Systems Used in the Treatment of BPH
KTP: Nd:YAG Lasers
By virtue of its selective absorption by hemoglobin, as well as its low penetration depth in tissue, the KTP laser vaporizes the surface layer of BPH tissue rapidly and hemostatically, with a 1–2 mm rim of coagulation [42]. As a result of these characteristics, the effect of the KTP laser is often referred to as photoselective vaporization of the prostate (PVP).
Technique
Photoselective vaporization of the prostate (PVP) became a viable option for treatment of BPH with the advent of a high-power, 80 W KTP laser. The system uses a side-firing glass fiber, which can be passed directly through either a 22.5 or 23 Fr continuous flow laser cystoscope or a standard 27 Fr continuous-flow resectoscope. Saline is the favored irrigant, which minimizes the risk of dilutional hyponatremia. The speed of tissue removal is a drawback with the PVP 80 W laser and is limited to 0.3–0.5 g/min [46]. This limits its use in larger prostates, which may take a considerable amount of time to complete the ablation.
The procedure is performed under general or spinal anesthesia, with the use of video endoscopy. Laser vaporization is accomplished by holding the laser fiber approximately 0.5–1 mm away from the tissue, while deploying a rotational motion over the adenoma. A 600 μm fiber with a 1.8 mm quartz capsule emits the beam at a 70° lateral deflecting angle [47].
The procedure usually begins at the bladder neck posteriorly. If there is a prominent median lobe, visual ablation of the obstructing adenomatous tissue can immediately be noted. By starting at this region, the operating surgeon allows for clearance of this tissue, which facilitates the passage of continuous-flow irrigant during the remainder of the procedure. The ureteral orifices should be noted at the outset, and great care should be exerted in avoiding injury to the bladder or either ureteral orifice.
Following ablation of the median lobe, similar to a TURP, the surgeon ablates the lateral lobes, the floor, and finally the apex. An attempt to vaporize tissue down to the level of the striated pseudocapsular fibers should be made. When arterial or venous bleeding is recognized, the laser can be moved away from the tissue, which defocuses the beam and allows for tissue coagulation. Alternatively a near contact technique can be used with lower power settings of 30–40 W [42].
Results
Following a series of single-center case reports [48, 49], Te et al. [50] reported on the results of the first multicenter prospective trial evaluating the 80 W KTP laser from the United States. With 139 men and 12-month follow-up, AUA-SI decreased from 23.9 to 4.3, QOL score decreased from 4.3 to 1.1, maximal urinary flow rate (Qm) increased from 7.8 to 22.6 ml/s, and the post-void residual urine volume (PVR) decreased from 114.3 to 24.8 ml. The transrectal ultrasound-estimated prostatic volume decreased from 54.6 to 34.4 ml, establishing this as a viable ablative option for select men with BPE. Approximately one third of patients did not require a catheter beyond the recovery room, and 86 % were hospitalized for less than 24 h. Complications occurred in 8.6 % of individuals and consisted of transient hematuria, dysuria, and urinary retention. At 1 year, two patients developed bladder neck contractures and one patient developed a soft urethral stricture.
Two randomized clinical trials (RCTs) comparing 80 W KTP with TURP have reported mixed results (Table 16.4). In the first, Bouchier-Hayes et al. [51] randomized 76 patients with medium-sized glands to KTP versus TURP. Overall KTP laser provided similar results to TURP, with respect to symptom score reduction, Qmax, and PVR. In the other RCT by Horasanli et al. [52], TURP significantly outperformed PVP in terms of all IPSS, Qm, and reduction of PVR.