Life on earth is based on genes that are passed on from one generation to the next. Genetic material is transmitted in the form of nucleic acids (DNA) contained within the chromosome, which is in the nucleus of the cell. Each time a cell divides, it creates a complete copy of itself. Any errors that occur while the genetic information is being replicated are termed mutations. Considering the extensive amount of data contained within DNA, such errors are unavoidable. For the most part, these small mutations have no physiologically significant effect, but in some cases the resulting change is incompatible with the cell’s life processes. In very rare cases, the mutation actually increases the new cell’s chances of survival over those of the original cell. Throughout many millions of years and countless generations, this process has cumulatively led to a huge diversity of species.
Seen over the course of generations, mutation is what drives evolution—but in an individual it can endanger health and even life. A genetic mutation that changes the physiological properties of an organism may have any of a number of effects. If the modified cell continues to “play by the rules” of the organism then the mutation generally presents no danger.
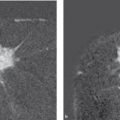
If a cell loses its characteristic property of living within a community of cells, however, then it has become what we call a cancer cell. Generally, a series of six to eight mutations is required before this point is reached. Specifically, cancer cells lack the property of “contact inhibition,” the trait that inhibits cell migration into other cells and organs (▶ Fig. 2.1). In a manner of speaking, contact inhibition is what regulates the different “territories” in multicellular organisms. It prevents cells from spreading into organs in which they do not belong.

Fig. 2.1 Tumor development. (a) A mutated cell clone (gray) has evolved within a milk duct from the epithelium (white). (b) Over time, a second (green) and a third (orange) mutation occur. (c) Further mutations in the course of time; e.g., up to a sixth mutation (red). (d) This cell clone has lost its contact inhibition and thus supplants the surrounding cells. (e) With further time, the cell clone has penetrated the boundaries of anatomical structures: it has become an invasive tumor.
The loss of contact inhibition makes it possible for a cancer cell to infiltrate foreign tissue. This process is aided by certain enzymes that dissolve connective tissue and other structures. Furthermore, cancer cells can leave their point of origin and move via lymph vessels and blood vessels. These processes are termed lymphatic spread and hematogenous spread, respectively, and can lead to the formation of metastases.
Cancer cells are characterized by an accelerated metabolism and an exceedingly rapid cell cycle. They thus have particularly high nutritional requirements. To meet their extensive needs, these cells secrete enzymes that stimulate the surrounding tissue to rapidly form a system of capillaries, a process termed angiogenesis or tumor angiogenesis. These new capillaries are morphologically simple endothelial tubes that lack the complex structure of normal blood vessels but suffice to enable the development of carcinoma cells and to supply them. This capillary network drains energy and nutrients from the surrounding organs, and consequently from the overall organism. Sometimes, however, cancer cells grow so rapidly that even the dedicated blood supply cannot feed all parts of the tumor, and cancer cells begin to die, usually in the center of the tumor.
2.2 Risk Factors
The probability that a woman will develop breast cancer at some point in her life depends on a number of different factors (▶ Table 2.1, ▶ Table 2.2). Oncogenes and tumor suppressor genes play an important role in the regulation of healthy cell growth and tumor development. Oncogenes accelerate cell growth, while tumor suppressor genes slow it down.
Risk of disease | Age group (years) | |||
25–44 | 45–54 | 55–79 | >80 | |
Absolute 5-year risk (%) | <0.5 | 0.5–1 | 1–1.5 | 1.5–2 |
Per 1,000 women | <5 | 5–10 | 10–15 | 15–20 |
Risk factors | Relative risk |
Cancer in childhood | 20 |
Radiotherapy (age 9–15 years) | 10 |
Mantle-field radiotherapy | 10 |
High-risk | 4.0–10 |
Type ACR IV tissue density | 3.8–5.2 |
Status post surgery for contralateral carcinoma: | |
| 5.0–9.0 |
| 3.7–4.1 |
| 1.8–3.0 |
Status post surgery for DCIS (premenopause) | 5 |
Status post surgery for ADH | 2.0–4.0 |
Menarche at age <11 years | 3 |
Menopause at age >54 years | 2 |
Abbreviations: ACR, American College of Radiology; ADH, atypical ductal hyperplasia; DCIS, ductal carcinoma in situ. | |
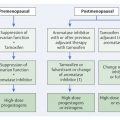
On average, a woman in Europe has an 11% chance of developing breast cancer. In industrialized countries, the incidence of breast cancer is rising and the average age of initial illness is dropping. In Asia and Africa, this risk is significantly lower, which suggests that certain lifestyle factors may play a significant role. In those parts of China, for example, where Western standards of living have been introduced, the incidence of breast cancer is increasing accordingly. One possible explanation for this is the decreased bodily production of melatonin as a result of the use of artificial light, which shortens the night phase. This results in an increased risk of breast cancer. A lower risk of breast cancer is seen among women who have late menarche or early menopause, and among those who bear and breast-feed children. The risk is increased, on the other hand, by such factors as obesity, alcohol consumption, and lack of physical exercise. Female hormone replacement therapy—in particular if hormones are taken for long periods—increases the risk of breast cancer 1.5-to 3-fold.
Take Home Points
Age and breast cancer risk: The most important population-based risk factor for breast cancer is increasing age.
Mammographic density and breast cancer risk: A high mammographic density (ACR III or IV) is one of the greatest individual risk factors.
2.3 Genetic Risk Factors
In addition to lifestyle-related factors, genetic factors can also affect a woman’s risk of breast cancer. In families with a high incidence of breast cancer, an individual’s lifetime risk of developing breast cancer can be as high as 80%. In these families there is also an increased incidence of ovarian carcinoma.
The high incidence of these cancers in high-risk families is due to defects in repair enzymes, which play an important role in cell division in both organs. These enzymes are proteins that check and correct the double-stranded DNA during the cell division process, normally reducing the number of mutations. If the genes coding for these repair enzymes are altered, then these enzymes can lose either the ability to detect replication errors or the ability to correct detected replication errors. These defective enzyme genes are called breast cancer (or BRCA) genes (▶ Table 2.3). Other genes associated with breast/ovarian cancer are RAD51C and RAD51D. It has also long been known that persons who have ataxia telangiectasia or Li–Fraumeni syndrome have a higher risk of breast cancer.
Gene | Percent lifetime risk of | |
| Breast carcinoma | Ovarian carcinoma |
BRCA1 (detectable) | 80 | 45 |
BRCA2 (detectable) | 80 | 20 |
BRCA3 (detectable) | ? | ? |
BRCA4 | ? | ? |
BRCA mutations are inherited in an autosomal dominant inheritance pattern: they can be inherited from father or mother. If one parent carries this mutated gene, each child has a 50% chance of inheriting it.