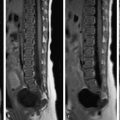
Masses of the spinal canal are classified by their anatomic location. They may involve the extradural, intradural–extramedullary, or intramedullary space. Localizing a mass to a specific compartment is often helpful in narrowing the differential diagnosis. Many masses affect multiple compartments, the most common pattern being extradural tumors with intradural involvement. Approximately 55% of tumors in adults are extradural, 40% are intradural, and 5% are intramedullary. Conventional radiographs have only a limited role in the diagnosis of spinal tumors, especially for preoperative imaging. Only 10% of spinal tumors show abnormalities on plain radiographs such as widening of the neural foramina, vertebral body scalloping, or calcifications. CT may be useful for the more precise evaluation of calcifications and osteolytic lesions. Today myelography has been almost completely replaced by MRI and is used only if there are contraindications to MRI. CT with intravenous contrast can provide an effective alternative: opacification of the extradural venous plexus can aid anatomic orientation and improve the delineation of masses. MRI is the imaging modality of choice, as its multiple planes and contrasts allow for the precise anatomic localization of a mass. The protocol should include sagittal T1w sequences, sagittal T2w and STIR sequences, and intravenous contrast administration as required. The sagittal slice thickness should not exceed 3 mm. Note Fat saturation should not be used after contrast administration for imaging intramedullary lesions, as it would increase image noise and could mask subtle findings. Fat saturation is recommended for extradural lesions, as it allows true enhancement to be distinguished from hyperintense fat. In patients with extradural lesions, it has been suggested that the unenhanced T1w sequence or STIR sequence could be omitted to save time. Both have comparable detection rates for intraosseous processes, but each can effectively complement the other and provide an internal control: if an osseous lesion is hypointense in the unenhanced T1w sequence and hyperintense in the STIR sequence, it is probably malignant. Pitfall Remember that the STIR sequence may yield false-negative findings after contrast administration, so it should always be performed before contrast injection. If the patient has complaints suggesting a lumbar mass but diagnostic studies do not reveal a mass in that region, the cervical and thoracic spine should also be imaged. The spinal extradural space contains all structures of the spinal column that are located outside the dural sac. It includes the osseous structures, ligaments, connective tissue, and intervertebral disks. The following tumors and tumorlike lesions may occur in the extradural space: Metastases. Hemangioma. Plasmacytoma. Lymphoma. Chordoma. Epidural lipomatosis. Osteosarcoma. Ewing’s sarcoma. Ganglioneuroma. Ganglioneuroblastoma. Neuroblastoma. Osteoid osteoma, osteoblastoma. Epidemiology and pathology Vertebral body hemangioma is one of the most common spinal masses. Approximately 12% of the population have at least one hemangioma. The great majority of hemangiomas are detected as incidental findings. They are not tumors in the true sense but congenital vascular malformations that are composed of cavernous, capillary, or venous vessels. Most hemangiomas are of the cavernous type. Clinical manifestations Approximately 1% of hemangiomas may cause clinical symptoms, doing so through either an extraosseous component or a pathologic fracture. Most of these patients are in the fourth to sixth decade of life. The thoracic spine is most commonly affected, followed by the lumbar and cervical spine. While asymptomatic hemangiomas occur with equal frequency in both sexes, symptomatic hemangiomas are more common in women. MRI findings Most hemangiomas are hyperintense on T1w images due to the elevated fat content of their vascular stroma. But there are also hemangiomas that do not have a high fat content, and they show intense enhancement ( ▶ Fig. 14.1d–f). Without contrast medium, they tend to be isointense on T1w images. Analogous findings are seen on T2w images ( ▶ Fig. 14.1b, c). Fig. 14.1 Hemangioma of the T9 vertebral body with a significant soft-tissue component and narrowing of the spinal canal in a 46-year-old man with increasing paraparesis. Decompressive laminectomy 3 years earlier afforded temporary improvement of complaints. (a) Axial CT scan shows a typical coarse trabecular pattern in the T9 vertebral body. The patient had a previous decompressive laminectomy. (b) Sagittal T2w sequence shows hyperintensity of the T9 vertebral body with a significant soft-tissue component impinging on the spinal cord. (c) Axial T2w sequence shows marked narrowing of the spinal canal and a paravertebral soft-tissue mass with para-aortic extension. Even MRI shows faint signs of a coarsened trabecular pattern. (d) Axial T1w sequence after contrast administration shows a prominent soft-tissue component with homogeneous enhancement. (e) Sagittal T1w sequence after contrast administration with spectral fat saturation. The vertebral body and soft-tissue component show marked, homogeneous enhancement. (f) Image after preoperative intervention. Initial transarterial embolization with particles, acrylic glue (Glubran), and silk was followed by percutaneous embolization with Glubran. Absence of enhancement indicates a significant decrease in tumor blood flow. Tips and Tricks A small number of hemangiomas may be located in the pedicle or extend into the pedicle from the vertebral body. These lesions require differentiation from malignancies. CT findings CT is an important tool for confirming the diagnosis. Hemangiomas are distinguished on CT by pronounced thickening and rarefaction of the bony trabecular markings, especially in the longitudinal direction ( ▶ Fig. 14.1a). Treatment Treatment may be appropriate for symptomatic hemangiomas. Given the rich vascularity of hemangiomas, surgical treatment should be preceded by embolization to reduce blood loss (see ▶ Fig. 14.1b). Transarterial embolization alone is helpful only in selected cases. Alternatives are direct percutaneous embolization or, for hemangiomas without a mass effect, vertebroplasty. Hemangiomas are also treatable by radiotherapy, although in published studies it took 1 to 18 months for this therapy to achieve pain reduction. Osteoid osteoma, osteoblastoma Both names refer to the same tumor. The main difference relates to size: tumors smaller than 2 cm are called osteoid osteomas. Pathology and epidemiology Histologically, these tumors consist of a nidus of osteoid tissue surrounded by a zone of reactive sclerosis. Osteoid osteomas are most commonly found in the posterior vertebral elements of patients younger than 30 years of age. They are usually located in the neural arch but may also occur in the vertebral body. Clinical manifestations and treatment Tumors that are symptomatic often cause nocturnal pain that responds well to salicylates. In many cases the symptoms are self-limiting over a period of several years. Osteoblastomas are more likely to cause radicular symptoms because of their size. The tumors may recur after surgical removal. CT and MRI findings As a rule, CT can clearly demonstrate the nidus and sclerosis. With MRI, a STIR sequence will often show pronounced bone edema and may show fluid in the surrounding soft tissues ( ▶ Fig. 14.2b). The lesion is usually hyperintense on T2w images. Because some tumors have extensive calcifications, low T2w signal intensity ( ▶ Fig. 14.2c) does not rule out osteoid osteoma. Contrast enhancement is frequently observed, especially in the surrounding soft tissue (flare phenomenon, ▶ Fig. 14.2a). Unenhanced T1w images show a hypo- to isointense signal ( ▶ Fig. 14.2a). Fig. 14.2 Osteoblastoma in the cervical spine. (a) The tumor shows unusually heavy calcification. It is markedly hypointense in the T1w sequence. (b) The tumor remains hypointense in the STIR sequence, but the hyperintense rim signifies a marked reaction of the surrounding tissue. (c) The tumor is also hypointense in the axial T2w sequence due to its heavy calcification. (d) Axial fat-saturated sequence after contrast administration shows moderate, inhomogeneous tumor enhancement with intense enhancement of the surrounding tissue (flare sign). Pitfall Some osteoid osteomas have a prominent perifocal reaction on MRI that may easily be mistaken for a malignant mass. Epidemiology and pathology Only 3 to 7% of giant cell tumors occur in the spinal column. Although they cause osteolytic destruction, these tumors are benign. The sacrum is a site of predilection in the spine. Giant cell tumors are not associated with peripheral sclerosis. The peak age incidence is between 10 and 40 years. Clinical manifestations and treatment Preoperative embolization should be considered due to the hypervascular stroma and the potential for heavy blood loss. The most common clinical manifestations are pain and fractures. Pain reduction was achieved in some studies by embolization with particles and cisplatin. Recurrence is common after an incomplete resection. Approximately 10% of giant cell tumors undergo malignant transformation. Initial metastasis generally occurs to the lung ( ▶ Fig. 14.3b). Fig. 14.3 Giant cell tumor in an 18-year-old woman with an atypical metastatic giant cell tumor in her right humerus. (a) Fat-saturated sagittal T1w image after contrast administration shows extensive skeletal metastasis in the spine with multiple enhancing masses and a compression fracture of the T12 vertebral body. (b) Extensive pulmonary metastases. MRI findings MRI shows a sharply circumscribed mass with low T1w and high T2w signal intensity. Postcontrast images usually show faint, inhomogeneous enhancement ( ▶ Fig. 14.3a). The soft-tissue component of these tumors may be very large. Intratumoral hemorrhage and shell-like calcifications are occasionally observed. Epidemiology and pathology Osteochondromas account for more than 30% of benign bone tumors and result from the separation of a cartilage fragment. Very rare cases (≤1%) undergo malignant transformation to chondrosarcoma. Histologically, the lesion is composed of normal bone and cartilage. Osteochondromas of the spinal column are rare (<5%), with the majority occurring in the cervical spine. Imaging findings Osteochondromas appear on conventional radiographs as bony projections on the surface of bones. On CT there is no discernible boundary between the tumor and normal bone. The cartilage cap may be calcified in varying degrees. Osteochondroma has intermediate signal intensity in both T1w and T2w sequences. Epidemiology Chondroblastomas most commonly occur in the tubular bones. The peak age incidence is 10 to 20 years. Chondroblastomas of the spinal column are rare (<2%) and are more likely to occur in older adults. MRI findings Chondroblastomas may occur in the vertebral arch or vertebral body. Chondroblastomas in tubular bones typically appear as an osteolytic area with scattered calcifications. The osteolytic area generally has sclerotic margins. Chondroblastomas of the spinal column are more aggressive in their behavior, appearing as expansile, destructive lesions. It is common to find associated narrowing of the spinal canal. Perifocal bone edema is generally absent. Signal characteristics range from hypointense to hyperintense, depending on the composition of the chondroblastoma. Contrast enhancement is consistently present. Tips and Tricks Chondroblastomas of the spine are virtually indistinguishable from chondrosarcomas on the basis of their imaging appearance. Patient age is an important clue: chondrosarcoma patients are generally older than patients with chondroblastoma. Epidemiology and pathology Aneurysmal bone cysts are rare and constitute only about 1% of all bone tumors. Less than 20% occur in the spinal column. The cause of aneurysmal bone cysts is unknown. Presumably it relates to osseous repair processes that leave behind a residual blood-filled cystic cavity. The posterior part of the vertebral body is a site of predilection ( ▶ Fig. 14.4). Fig. 14.4 Aneurysmal bone cyst in a 13-year-old girl with back pain of sudden onset and a cystic mass at the L1 level. (a) Sagittal T2w sequence. (b) Axial T2w sequence. (c) Coronal STIR sequence. Treatment Recurrence is common after an incomplete resection (up to 30% of cases). Transarterial embolization should be considered preoperatively due to the high bleeding risk from the very vascular lesion. Embolization is occasionally therapeutic in itself, as it can halt lesion expansion and relieve pain. Differential diagnosis Aneurysmal bone cysts can usually be differentiated from giant cell tumors based on patient age: aneurysmal bone cysts are most common before 20 years of age, whereas giant cell tumors tend to occur after age 20. Fluid levels are often present in aneurysmal bone cysts. This is not a pathognomonic feature, however, and may occur in other tumors such as osteosarcomas, chondroblastomas, and giant cell tumors. Epidemiology Eosinophilic granuloma, like Hand–Schüller–Christian syndrome and Abt–Letterer–Siewe syndrome, is a granulomatous disease that is a form of Langerhans cell histiocytosis. Approximately 7% of eosinophilic granulomas occur in the spinal column. The disease is most common in patients under 30 years of age and predominantly affects the thoracic spine in this age group. Clinical manifestations The clinical hallmarks of eosinophilic granuloma are pain and limited motion. Compression fractures are common and may be the initial presenting symptom. Vertebra plana in children should always raise suspicion of eosinophilic granuloma but is not pathognomonic as it is also found with malignant lesions such as Ewing’s sarcoma. MRI findings A vertebral body affected by eosinophilic granuloma typically shows a heterogeneous structure with decreased vertebral height. A perifocal soft-tissue reaction is present in one-third of cases. The lesion usually has low T1w signal intensity and high T2w signal intensity. Pathology Strictly speaking, epidural lipomatosis is a tumor but not a neoplasm. It involves an excessive accumulation of fatty tissue which, unlike a lipoma, is not encapsulated. Possible causes include steroid therapy, obesity, or Scheuermann’s disease. Often a cause cannot be established, however. Borré classified epidural lipomatosis of the lumbar spine into four grades based on the ratio of dural sac to fat: grade 0 (ratio ≥1.5) and grades 1 to 3 (ratio from 0.33 to <1.5). Grade 3 may produce symptoms like those of spinal stenosis. For the most part, however, this classification shows little correlation with clinical manifestations and therefore has not been widely adopted. MRI findings The fat deposition in epidural lipomatosis is often located posterior to the spinal cord, with a predilection for the thoracic region ( ▶ Fig. 14.5). Differentiation from hematoma is occasionally difficult but is aided by acquisition of a fat-saturated sequence ( ▶ Fig. 14.6). Fig. 14.5 Epidural lipomatosis. (a) Sagittal T1w image. (b) Axial T1w image shows pronounced epidural fat deposition in this obese patient. Fig. 14.6 Epidural lipomatosis in a different patient. Pronounced epidural lipomatosis is apparent in the T1w and STIR sequences. (a) T1w image. (b) STIR image. (c) Image with spectral fat saturation aids differentiation from epidural hematoma. Like intracranial arachnoid cysts, spinal arachnoid cysts are not neoplasms. Generally they are incidental findings but in rare cases may lead to pain syndromes or deficits. Anatomically, arachnoid cysts represent the protrusion of arachnoid membrane through defects in the dura. They are often located in the thoracic or lumbar region ( ▶ Fig. 14.7) and may be congenital or acquired. Pressure from an arachnoid cyst may deform the adjacent bone. If surgery is indicated, preoperative myelography may be appropriate. It can define the extent to which the cyst communicates with the “free” subarachnoid space and can help direct surgical planning. Fig. 14.7 Lumbosacral extradural arachnoid cyst. (a) Sagittal T1w image. (b) Sagittal STIR image. (c) Sagittal gadolinium-enhanced T1w image. (d) Axial T2w image. Epidemiology and pathology The majority of malignant spinal masses are metastatic. Up to 40% of all patients with a malignant underlying disease will eventually develop spinal metastases, predominantly in the vertebral bodies. The incidence of vertebral body metastasis increases with patient age: pediatric tumors rarely metastasize to the vertebrae. The leading sources of spinal metastases are breast cancer, lung cancer, and prostate cancer. An osteolytic growth pattern is the most common. Osteoplastic metastases may occur with prostate cancer, certain forms of breast cancer, lymphomas, and carcinoids. MRI findings Osteolytic metastases are generally characterized in older patients by low T1w signal intensity due to displacement or infiltration of the fatty marrow. Thus, osseous lesions that are hypointense in T1w sequences are considered suspicious for malignancy. This particularly applies to multifocal lesions. The differential diagnosis should include activated osteochondrosis or fractures, which may have a similar appearance. Osteolytic metastases are hyperintense in T2w sequences, while osteoplastic metastases are hypointense due to new bone formation. Note The unenhanced T1w sequence alone is superior to a technetium bone scan for diagnosing spinal metastasis. Not only does it provide better spatial resolution, but the fat marrow infiltration demonstrable by MRI can detect the lesion before osseous changes become apparent on bone scans. Bone metastases that are hypointense on unenhanced images may be masked by contrast administration, as enhancement causes the lesions to become isointense. Nevertheless, contrast administration is still advised because it improves the visualization of extraosseous tumor components. Fat-saturated T1w sequences can display the enhancement with exceptional clarity. There is debate as to whether unenhanced T1w sequences or STIR sequences provide a better detection rate for bone metastases ( ▶ Fig. 14.8, ▶ Fig. 14.9). Ultimately, however, a combination of both sequences is probably the best approach as it can define both the core lesion and perifocal edema. It should be noted that a STIR sequence performed after contrast administration may lead to false-negative findings. It may also be helpful to add a diffusion sequence, especially since diffusion sequences are now available that are much less susceptible to artifacts. Neoplastic fractures often show restricted diffusion whereas traumatic and osteoporotic fractures do not. Fig. 14.8 Osteoplastic metastases from breast cancer. Extensive osteoplastic metastasis from breast cancer in a 43-year-old woman. (a) Sagittal STIR image. (b) Sagittal unenhanced T1w image. Fig. 14.9 Osteolytic metastases from lung cancer. Pronounced osteolytic metastases in the axial skeleton of a 55-year-old man. (a) Sagittal STIR sequence shows distinct osseous hyperintensities. (b) Unenhanced T1w sequence shows corresponding hypointensities. (c) Fat-saturated sagittal T1w sequence after gadolinium administration shows marked enhancement. Multiple myeloma and plasmacytoma are not synonymous terms, as is often believed, but refer to different stages of the same disease. “Plasmacytoma” denotes a solitary lesion, which may occur at an extraosseous site. “Multiple myeloma” refers to multifocal disease. Solitary lesions are observed in no more than 5% of monoclonal gammopathies. MRI findings Multiple myeloma The peak age incidence of multiple myeloma is the sixth decade. The vertebral bodies are commonly affected by multiple separate lesions. Other cases show diffuse infiltration, which may be difficult to detect due to an absence of visible healthy bone. The lesions have low T1w signal intensity ( ▶ Fig. 14.10b) and are hyperintense on T2w and STIR images ( ▶ Fig. 14.10a). Epidural seeding is very common and may lead to long segmental narrowing of the spinal canal with cord compression. Pathologic fractures may also occur. Contrast medium is unnecessary for the visualization of intraosseous changes but is useful for defining the extraosseous component. Fat-saturated T1w sequences are helpful in this regard ( ▶ Fig. 14.10c). Fig. 14.10 Multiple myeloma of the lumbar spine. (a) Sagittal STIR sequence. Hyperintensities with marked involvement of the lumbar spine. (b) Unenhanced T1w sequence. (c) T1w sequence with gadolinium and spectral fat saturation shows multiple enhancing areas in the affected vertebral bodies. Plasmacytoma Conventional radiographs are still used for evaluating skeletal involvement by plasmacytoma, but studies have shown that MRI is much more sensitive than plain radiography in detecting plasmacytoma lesions. Diffusion sequences in particular can be used to image the whole body in a reasonable time frame. Unfortunately, MRI has not yet been incorporated into official guidelines for the diagnosis and treatment of plasmacytoma, and therefore conventional imaging continues to play a role. Differential diagnosis It is important to distinguish between metastasis and plasmacytoma on the basis of their imaging morphology. Approximately 80% of plasmacytomas show a distinctive MRI pattern marked by hypointense lines in the vertebral body, which are thought to represent thickened trabeculae. Scalloping of the vertebral body cortex may also be observed. MRI findings Non-Hodgkin’s lymphoma is the type that most commonly affects the spinal column. Leukemic infiltrates (chloromas) may also occur; the two conditions are indistinguishable by their imaging appearance. Leukemic infiltrates and Hodgkin’s disease are more common in children and adolescents, while non-Hodgkin’s lymphoma is more prevalent in older adults. MRI often shows normal vertebral bodies embedded in an enhancing soft-tissue mass. Frequently the mass is in contact with the retroperitoneal space or mediastinum. Infiltration of the epidural space and neural foramina is also common, with a potential for associated compression syndromes. Cortical bone destruction is found in 35% of patients with lymphoma or leukemia, versus more than 75% of patients with metastasis or carcinoma. The presence of concomitant lymph node enlargement makes the diagnosis very likely. Analogous to multiple myeloma, diffuse involvement of the spinal column by lymphoma is a domain of MRI, which can detect signal changes before osseous reactions have occurred. Bone marrow infiltration typically leads to a decreased fat signal with resulting hypointensity or a hyperintense signal in the STIR sequence ( ▶ Fig. 14.11). The MRI findings in these cases are even superior to biopsy. Fig. 14.11 Non-Hodgkin’s lymphoma of the upper thoracic spine in a 56-year-old man with a pronounced epidural soft-tissue component and destruction of the T3 vertebral body. (a) Sagittal T2w image. (b) Sagittal STIR image. (c) Sagittal unenhanced T1w image. (d) Sagittal contrast-enhanced T1w image with spectral fat saturation. (e) Axial contrast-enhanced T1w image with spectral fat saturation. Epidemiology and pathology Chordomas arise from embryonic remnants of the notochordal tissue from which the vertebral bodies and intervertebral disks are formed. They are particularly common at the ends of the spinal column (clivus and sacrum). All age groups are equally affected. MRI findings Chordomas typically show high T2w signal intensity ( ▶ Fig. 14.12) due to the high fluid content in the matrix of this osteolytic tumor. Fig. 14.12 Chordoma of the T12 vertebral body in a 58-year-old man with an increasingly unsteady gait and hip flexor weakness. The hyperintense T2w signal with a disordered, heterogeneous pattern is a typical finding. The marked contrast enhancement in this case is somewhat unusual. (a) Sagittal STIR sequence. (b) Sagittal T1w sequence. (c) Sagittal T2w sequence. (d) Contrast-enhanced T1w sequence with spectral fat saturation.
14.2 Extradural Space
14.2.1 Benign Tumors
14.2.1.1 Hemangioma


14.2.1.2 Giant Cell Tumor

14.2.1.3 Osteochondroma and Cartilaginous Exostosis
14.2.1.4 Chondroblastoma
14.2.1.5 Aneurysmal Bone Cyst

14.2.1.6 Eosinophilic Granuloma
14.2.1.7 Epidural Lipomatosis


14.2.1.8 Extradural Arachnoid Cyst

14.2.2 Malignant Tumors
14.2.2.1 Metastases


14.2.2.2 Multiple Myeloma and Plasmacytoma

14.2.2.3 Lymphoma

14.2.2.4 Chordoma

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree