8 Ultrasound assessment of the extracranial cerebral circulation
Symptoms of carotid and vertebral artery disease
Selection of treatment for carotid artery disease
Combining B-mode, color imaging, and spectral Doppler information
Normal and abnormal appearances of vertebral artery flow
Problems encountered in imaging carotid artery flow
Postoperative carotid artery appearance on ultrasound
Post carotid artery angioplasty and stenting appearance on ultrasound
Nonatheromatous carotid artery diseases
Transcranial Doppler ultrasound
INTRODUCTION
Ultrasound can be used to evaluate the extracranial cerebral circulation in order to investigate patients who may be at risk of suffering a stroke (patients who have suffered a transient ischemic attack or TIA) or who have already suffered a stroke. Stroke is the third most common cause of death in the UK, with the stroke rate being approximately 2 in 1000 of the population per year. Approximately 80% of strokes are ischemic (i.e., thrombotic or embolic or both) as opposed to hemorrhagic. Up to 80% of ischemic strokes occur in the carotid territory, the area of the brain supplied by the carotid arteries. Trials have shown that patients with significant carotid artery disease and relevant symptoms benefit from surgery in order to prevent a stroke. The majority of carotid artery disease develops at the carotid bifurcation, and in the presence of a significant stenosis, carotid endarterectomy (CEA) can be performed. In this procedure, the diseased inner wall of the artery is removed, thus eliminating a potential source of emboli or flow-limiting stenosis. Carotid ultrasound examinations can be used to screen patients for carotid artery disease before further investigation. Alternatively, many centers now use ultrasound examination to select patients directly for surgery, without preoperative angiography, as angiography is known to carry its own small risk of transient and permanent neurological deficit. If the ultrasound scan is inconclusive and further imaging is required, magnetic resonance angiography (MRA) or computed tomography angiography (CTA) may make safer alternatives to X-ray angiography for confirming ultrasound findings prior to surgery or for further investigations, when ultrasound has provided only limited results. Some centers perform CEA for significant carotid disease prior to or combined with coronary artery bypass graft (CABG) surgery, with the aim of reducing the stroke rate associated with CABG surgery. In these centers the cardiologist or cardiac surgeons may require a carotid disease screening service to detect the presence of any significant disease.
ANATOMY
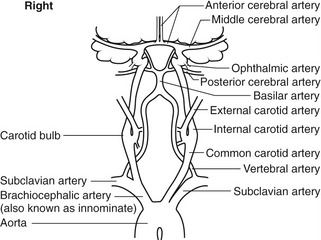
The brain is supplied by four vessels – the right and left internal carotid and vertebral arteries – and receives 15% of the cardiac output. The term extracranial cerebral arteries refers to all the arteries that carry blood from the heart up to the base of the skull. The left and right sides of the extracranial circulation are not symmetrical (Fig. 8.1). On the left side, the common carotid artery (CCA) and subclavian artery arise directly from the aortic arch, whereas on the right side the brachiocephalic artery, also known as the innominate artery, arises from the aorta and divides into the subclavian artery and CCA. The CCA, which has no branches, divides into the internal and external carotid arteries (ICA and ECA, respectively), but the level of the carotid bifurcation in the neck is highly variable. In approximately 90% of cases, the ICA lies posterolateral or lateral to the ECA and, unlike the ECA, has no branches below the skull. The proximal branches of the ECA are the superior thyroid, lingual, facial, and maxillary arteries. The carotid artery widens, at the level of the bifurcation, to form the carotid bulb. In some cases, the carotid bulb may only involve the proximal ICA, and not the distal CCA, and the degree of widening of the carotid bulb is quite variable. Within the skull, the distal segment of the ICA follows a U-shaped curved path, known as the carotid siphon. The most important branch of the ICA is the ophthalmic artery, which supplies the eye. The terminal branches of the ophthalmic artery, the supratrochlear and supraorbital arteries, unite with the terminal branches of the ECA. The ICA finally divides into the middle cerebral artery (MCA) and the anterior cerebral artery (ACA).
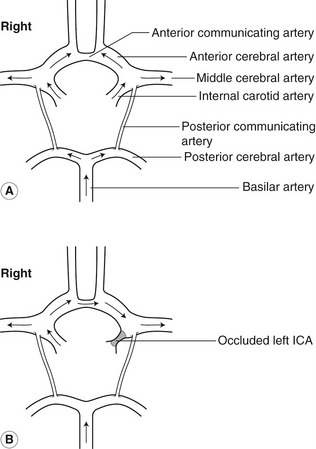
The posterior circulation of the brain is mainly supplied by the left and right vertebral arteries, via the basilar artery. The vertebral artery is the first branch of the subclavian artery, arising from the highest point of the subclavian arch. At the sixth cervical vertebra, the vertebral artery runs posteriorly to travel upward through the transverse foramen of the cervical vertebrae. It is common for one vertebral artery to be larger than the other, with the left often being larger than the right. The two vertebral arteries join, at the base of the skull, to form the basilar artery, which then divides to form the posterior cerebral arteries. Figure 8.2A shows how the circle of Willis, situated at the base of the brain, joins the cerebral branches of the ICAs and basilar artery via the anterior and posterior communicating arteries. Blood flow to the brain is regulated by changes in cerebrovascular resistance, with carbon dioxide playing a major role in vasodilation.
Collateral pathways and anatomical variants
The ECAs do not normally supply blood to the brain, but in the presence of severe ICA disease, branches of the ECA can act as important collateral pathways. One such pathway is via the terminal branches of the ECA, communicating with the terminal branches of the ophthalmic artery. This collateral pathway can be observed using continuous-wave (CW) Doppler to detect reversal of flow in the supraorbital artery, a terminal branch of the ophthalmic artery, as retrograde flow travels from the ECA branches toward the brain.
In the normal circulation, there is little blood flow through the communicating arteries in the circle of Willis, but in the presence of severe vascular disease they perform an important role in flow distribution. For example, in the presence of a left ICA occlusion, it is possible for the right ICA to supply blood flow to the left MCA via the right ACA, the anterior communicating artery and the left ACA, with flow reversal occurring in the left ACA (Fig. 8.2B).
The vertebral arteries may also supply flow to the MCA via the posterior communicating arteries of the circle of Willis. If the circle is well developed, it is possible for a single extracranial artery to provide adequate cerebral blood flow. However, in about 75% of the population, parts of the circle may be hypoplastic (very small) or absent, making the circle incomplete and therefore preventing the development of good collateral flow (von Reutern & von Büdingen 1993), but this may only become apparent in the presence of severe disease. Adequate collateral pathways have a better chance of developing in the presence of slowly developing disease.
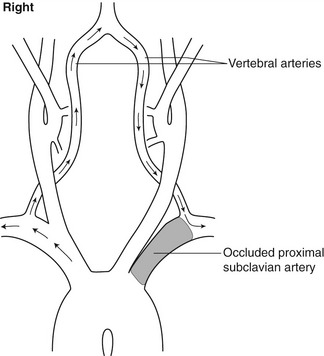
An unusual collateral pathway can occur when the CCA is occluded and flow in the proximal ECA reverses, being supplied by retrograde flow in an ECA branch, to supply a patent ICA. Severe narrowing or occlusion of the proximal subclavian or brachiocephalic artery can result in a collateral pathway that ‘steals’ blood from the brain to supply the arm. In this case, blood will be seen to flow retrogradely down the ipsilateral vertebral artery to supply the distal subclavian artery beyond the diseased segment (Fig. 8.3). This is known as subclavian steal syndrome.
There are few variations in the extracranial circulation. In rare cases, the left CCA and subclavian artery may share a common origin or a single trunk. Other anomalies are the left vertebral artery arising directly from the aortic arch and, even more unusually, the right vertebral origin arising from the aortic arch.
SYMPTOMS OF CAROTID AND VERTEBRAL ARTERY DISEASE
As the right side of the brain controls the left-hand side of the body and the converse, the symptoms will relate to the contralateral carotid artery. Speech is usually controlled by the dominant side of the brain (i.e., a right-handed patient’s speech will typically be controlled by the left side of the brain). Patients suffering from episodes of amaurosis fugax often complain of ‘a curtain drawing across one eye’ lasting for a few minutes, which is due to emboli within the retinal circulation. In this situation, the symptoms in the eye will relate to the ipsilateral carotid artery. Typical vertebrobasilar symptoms are shown in Box 8.1. Vague symptoms, such as dizziness and blackout, are not usually associated with carotid artery disease. Subclavian steal syndrome does not usually cause significant symptoms. Only about 15% of patients suffer symptoms of TIA before a stroke. Fifty percent of ischemic carotid territory strokes are due to thromboembolism of the ICA, whereas 25% are due to small-vessel disease and 15% are due to emboli originating from the heart. Only 1–2% of all strokes are hemodynamic strokes (i.e., due to flow-limiting stenoses) (Naylor et al. 1998).
BOX 8.1 Typical carotid territory and vertebrobasilar symptoms
(after Naylor et al. 1998, With permission)
Patients with symptoms of TIA or minor stroke are at a cumulative recurrent risk of a stroke due to large-vessel disease of 4% at 7 days following symptoms, 12.6% at 1 month, and 19.2% at 3 months (Naylor 2008). In other words, about 20% of people suffering a TIA will have a stroke within four weeks. Ultrasound can be used to help select those patients who would benefit from CEA to reduce the risk of stroke (method discussed later in the chapter). It has been shown that the benefit of CEA is greatest when the surgery is performed within 2 weeks of the patient’s symptoms and that the benefit is reduced by almost a third if surgery is performed more than 4 weeks following the last symptom (Rothwell et al. 2004). In symptomatic patients with a 70–99% stenosis, the absolute risk reduction of CEA is 23% if surgery is performed within 2 weeks compared to 7.4% if performed at more than 12 weeks after symptoms. Guidelines from the American Heart Association/American Stroke Association (Sacco et al. 2006) suggest that patients suffering from TIA or minor stroke should be assessed and, if appropriate, have surgery within 2 weeks. This, therefore, requires patients suffering TIA or minor stroke to have rapid access to carotid duplex scanning to enable early diagnosis and treatment.
SCANNING
Objectives and preparation
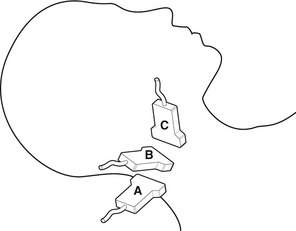
No specific preparation is required, but the patient must be capable of lying or sitting still during the examination. The optimal position for scanning the carotid arteries is with the sonographer sitting behind the patient’s head. This allows easy access to the neck and enables the operator to rest the arm on the examination table while performing the scan (Fig. 8.4). Alternatively the sonographer can sit by the side of the patient while resting the arm on the patient’s upper chest. The patient should lie supine on the couch with the head resting on a pillow. The neck should be extended and the head turned in the opposite direction to the side being examined. If the patient has difficulty in breathing or has back problems it may be necessary to sit the patient in a more upright position. If the patient is in a wheelchair (e.g., following a disabling stroke), it may be easier to do the scan in the wheelchair with the head resting on a pillow for support, preventing unnecessary movement of the patient. However, being in an upright position may affect the velocity values recorded and more care may be required in grading any significance disease (Pemble 2008).
Technique
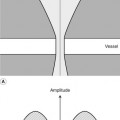
The carotid arteries are best visualized through the sternocleidomastoid muscle, which provides a good ultrasonic window, and this is done using a lateral rather than an anterior approach. The procedure is as follows:
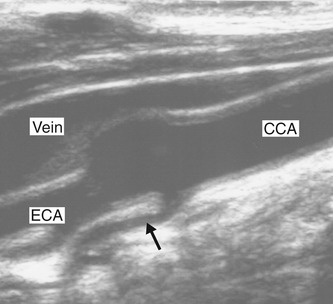
1. Using B-mode imaging only, the CCA should be visualized in transverse section (Fig. 8.5A), starting at the base of the neck. On the right side, it is usually possible to visualize the distal brachiocephalic artery and the origin of the CCA and subclavian arteries. On the left side, the origin of the CCA cannot be visualized as it lies too deep in the chest. The CCA should be scanned along its length, in transverse section, up to the bifurcation, and along the ICA and ECA (Fig. 8.5B) as high up the neck as can be seen. This allows the sonographer to ascertain the level and orientation of the carotid bifurcation and also gives the first indications of the presence and location of any arterial disease. The jugular vein lies over the CCA (Fig. 8.5A) and is usually easily compressed. However, it is important not to apply too much transducer pressure when scanning the carotid arteries as there is a possibility of dislodging an embolus from the vessel wall.
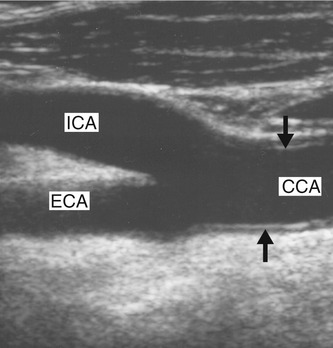
2. The CCA is now visualized in longitudinal section using B-mode imaging, starting at the base of the neck. A longitudinal image of the CCA can be easily obtained by imaging the CCA in transverse section and then, keeping the CCA in the center of the image, rotating the probe so the CCA first appears as an ellipse and finally can be seen in longitudinal section. Prior knowledge of the orientation of the ICA and ECA gained from transverse imaging is helpful for locating the correct longitudinal imaging plane to view the bifurcation. It is necessary to use a range of longitudinal scan planes to visualize the carotid arteries, especially at the bifurcation (Fig. 8.6). Typically, the ICA lies posterolateral or lateral to the ECA and is usually the larger of the two vessels. In a small percentage of cases, the bifurcation will appear as a tuning fork arrangement (Fig. 8.7), but in the majority of cases the ECA and ICA will not be seen in the same plane and will have to be imaged individually. This is achieved by keeping the lower portion of the probe face over the CCA and slowly rotating the upper portion through a small angle to image first the ICA and then the ECA, or vice versa. Only small probe movements are required when imaging the ICA and ECA, as the vessels usually lie close together.
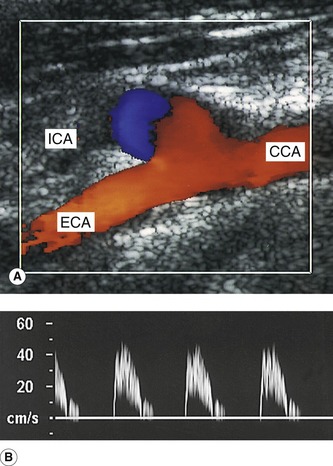
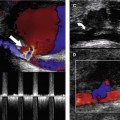
3. Having located the three vessels and observed any evidence of disease in the B-mode image, color flow imaging can be used to investigate the flow from the proximal CCA up into the ICA and ECA. Identification of ECA branches (either on B-mode or color imaging) serves as a further indication as to which vessel is the ECA, as the ICA has no branches below the jaw (Fig. 8.8). Color flow imaging can provide evidence of disease, such as velocity changes due to stenosis, areas of filling defects due to the presence of atheroma, and the absence of flow due to occlusion. Diagnosis should not be made based on the color flow imaging alone, but it greatly aids the sonographer in selecting areas that require close investigation with the spectral Doppler.
4. The spectral Doppler is now used to observe the inflow to the carotid arteries by placing the sample volume in the proximal CCA at the base of the neck. The shape of the waveform may reveal the presence of proximal or distal disease, such as an ICA occlusion. In the absence of significant distal or proximal disease, the left and right CCA waveforms should appear symmetrical.
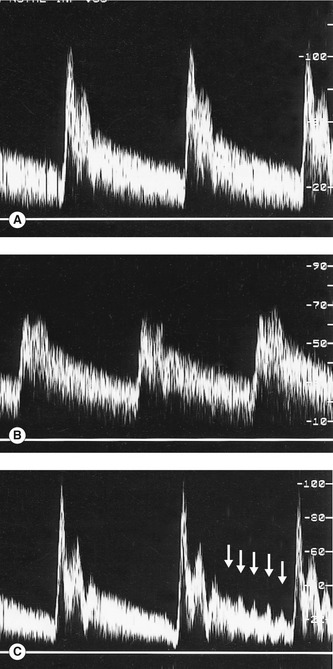
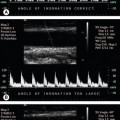
5. The examination so far has provided many clues as to which of the two vessels beyond the bifurcation is the ICA, such as the relative size and position of the two vessels and the presence of ECA branches. Spectral Doppler can now be used to confirm the identification of the ICA and ECA, as the ICA waveform shape is less pulsatile and has higher diastolic flow than the ECA (Fig. 8.9). Differentiation of the vessels may be further helped by tapping the temporal artery, an ECA branch (which runs in front of the upper part of the ear), as this will cause changes in the ECA flow during diastole (Fig. 8.9C) but will have little effect on the ICA. It is imperative that the ICA and ECA should be correctly identified, as it is the presence of disease in the carotid bifurcation and ICA, not the ECA, that is the possible cause of carotid artery symptoms. If significant disease is present in the ICA, the upper limit of the disease in relation to the level of the jaw should be assessed. If no clear vessel can be seen beyond the stenosis, angiography may be required to confirm the endpoint of the disease.
6. Using spectral Doppler, peak systolic and end-diastolic velocity (EDV) measurements should be made in the CCA, ICA, and ECA and at the site of the maximum velocity increase within any stenoses to allow the degree of narrowing to be graded. Atypical waveform shapes should also be noted.
7. If no flow is detected in the ICA (Fig. 8.10) or CCA using the default carotid preset scanner settings, it is necessary to rule out the presence of low-volume flow due to a critical stenosis or subtotal occlusion (Fig. 8.11) before reporting the vessel to be occluded. This is achieved by optimizing the scanner controls to detect low-velocity flow (i.e., by lowering the pulse repetition frequency [PRF] and high-pass filter setting). If low-velocity flow is detected, the cause should be identified. For example, low-velocity flow may be detected in the CCA because of an ICA occlusion (Fig. 8.10B), or it may be detected in the ICA due to a severe stenosis of the ICA origin.
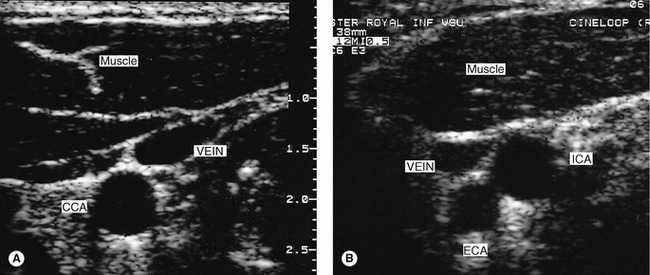
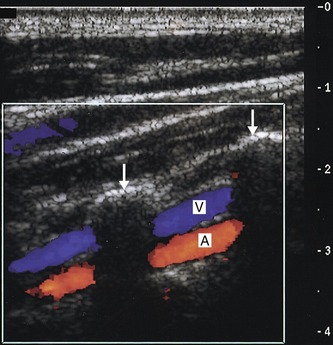
8. To conclude the first side of the examination, the vertebral artery should be located using B-mode or color imaging. The patient’s head should be turned slightly to one side. First image the mid-CCA in longitudinal section and then slowly angle the transducer into a more anteroposterior plane. The vertebral processes, seen as bright echoes, should slowly be seen to stand out. Only short sections of the vertebral artery and vein can be seen at this level as they run through the transverse foramen of the vertebrae. The walls of the vertebral artery and vein can often be seen on the B-mode image, but color flow imaging can also help visualize the vessels (Fig. 8.12). Spectral Doppler is then used to confirm the direction and quality of flow in the vertebral artery.
9. Having completed the first side of the examination, the patient is asked to turn the head in the opposite direction, and the other side is examined in the same way. It is important to remember that the carotid and vertebral arteries on both sides are linked via several possible collateral pathways and that the presence of severe disease in one extracranial vessel may affect flow in another extracranial vessel if it is supplying a collateral pathway.
B-MODE IMAGING
Normal appearance
The normal vessel walls will often appear as a double-layer structure when imaged in longitudinal section (Fig. 8.7), especially if a high-frequency transducer is used. This represents the intima-media layer and adventitia (Ch. 5) and is most clearly seen on the posterior wall in the CCA, when the vessel lies at right angles to the ultrasound beam. The normal thickness of the intima-media layer is of the order of 0.5–0.9 mm, when measured on ultrasound. A normal vessel lumen should appear hypoechoic; however it is possible for the sonographer to remove echoes from within the lumen by reducing the time gain compensation (Ch. 2), so careful use of the imaging controls is important. The B-mode imaging focal zone should be placed in the region of the vessel to insure optimal imaging of the vessel walls. Occasionally, it is difficult to obtain adequate B-mode images of the bifurcation. In this case, color flow imaging may help locate the vessels and enable spectral Doppler measurements to be made.