Fig. 25.1
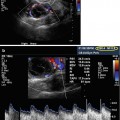
Transvaginal ultrasound: early intrauterine gestational sac, “the intradecidual sign”
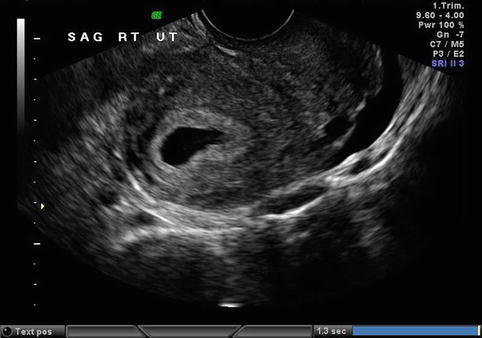
Fig. 25.2
Transvaginal ultrasound: intrauterine pseudosac associated with an ectopic pregnancy
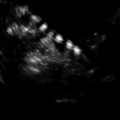
The yoke sac is the first visible structure within the gestational sac and is a distinct circular structure with a bright echogenic rim and sonolucent center (Fig. 25.3) and is recognized 3 weeks post-conception (5 weeks after the last menstrual period). The embryo is first recognized as a thickening along an edge of the yoke sac (Fig. 25.4), and embryonic cardiac motion can be first observed 3 1/2 to 4 weeks post-conception (5 1/2–6 weeks after last menstrual period). When exact pregnancy dating is available, an intrauterine pregnancy, regardless of embryonic number, should be identified within the endometrial cavity with transvaginal ultrasonography by 24 embryonic days or 38 menstrual days (exact 28 day menstrual cycle). This exact pregnancy dating does not rely on human chorionic gonadotropin, hCG, levels. Without such exact pregnancy dating and with no intrauterine pregnancy identified with transvaginal sonography, the “nondiagnostic ultrasound,” a serum level of hCG is needed for ultrasound interpretation [6]. A word of caution, because of the variation in vaginal ultrasound technical and interpretive abilities and lab hCG levels, before embarking on treatment for a presumed ectopic pregnancy, especially with methotrexate, give every pregnancy the “benefit of the doubt.” Be certain of the diagnosis or use diagnostic laparoscopy for confirmation.
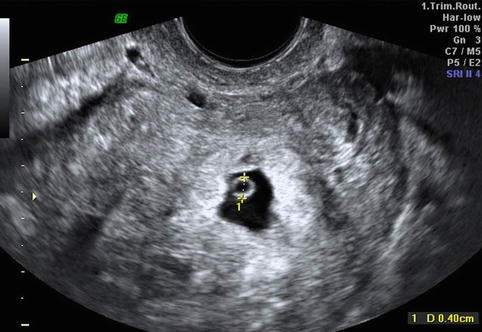
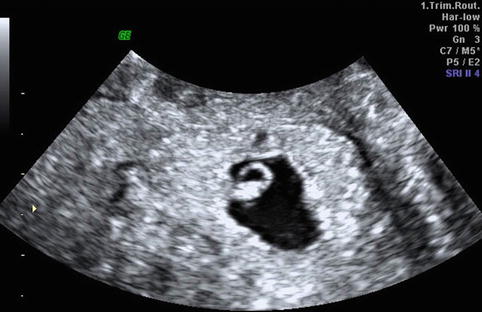

Fig. 25.3
Transvaginal ultrasound: intrauterine gestational sac containing a yolk sac

Fig. 25.4
Transvaginal ultrasound: thickened edge of a yolk sac representing an early developing embryo (embryonic cardiac activity may be present)
Additional information can be gained from transvaginal ultrasound measurement of the endometrial echo in early gestation, before the recognition of a gestational sac. Spandorfer and Barnhart reported statistically different endometrial echo thicknesses between patients with normal intrauterine, failed intrauterine, and ectopic gestations [7]. Patients with normal pregnancies had endometrial echo thicknesses of 13.42 ± 0.68 mm. In contrast, those with failed intrauterine and ectopic gestations measured 9.28 ± 0.88 and 5.95 ± 0.35 mm, respectively (P < .01). In this report, 97 % of patients with an echo no greater than 8 mm had abnormal pregnancies, and 71 % of these abnormal pregnancies were ectopic in location. Only 41 % of those patients with an echo thickness greater than 8 mm were abnormal, and only 14.7 % were ectopic in location. No patient with an endometrial echo thickness greater than 13 mm had an ectopic pregnancy, and no patients with an echo thickness less than 6 mm had a normal pregnancy. These are well-stratified differences, but other authors have seen much more overlap with endometrial echo measurements.
Usually, the transvaginal ultrasound identification of an intrauterine pregnancy reliably excludes an extrauterine implantation, except in the case of heterotopic pregnancy: the coexistence of an extrauterine implantation with an intrauterine pregnancy. The natural occurrence of heterotopic pregnancy is 1 in 4,000 pregnancies, but the frequency is much greater with pregnancies conceived with assisted reproductive technologies. Should a clinical presentation or abnormal pelvic ultrasound appearance suggest an ectopic pregnancy, despite visualization of an intrauterine gestation, the diagnosis of heterotopic pregnancy should be considered, with the probable need for diagnostic laparoscopy confirmation and treatment.
The possibility of ectopic pregnancy is frequently considered before hCG has reached the discriminatory zone and before ultrasound recognition [8]. Human chorionic gonadotropin rises exponentially in early normal pregnancy and should rise at least by 53 % in 48 h [9]. This exponential rise is less reliable after 10,000 mIU/ml, and at this level, pregnancy is better evaluated with ultrasound. Fifteen percent of normal intrauterine pregnancies can demonstrate an abnormal early rise of hCG, but for the majority of gestations, when the hCG rise is abnormal, at a plateau, or falling, an abnormal pregnancy is confirmed but not its location [10].
Cervical Pregnancy
Less than 1 %, and the rarest, of ectopics are implanted within the cervical canal below the level of the internal cervical os [11, 12]. The etiology of such implantations is unknown, but predisposing factors include prior uterine curettage, induced abortion, Asherman’s syndrome, leiomyomata, presence of an intrauterine device, in vitro fertilization, and prior in utero exposure to diethylstilbestrol [13–16].
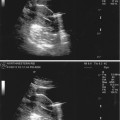
Before the now common use of early pregnancy transvaginal ultrasound, cervical pregnancies were frequently diagnosed at the time of spontaneous abortion or reached the second trimester, both associated with life-threatening hemorrhage frequently requiring hysterectomy as treatment. Usually, the first complaint is painless vaginal bleeding and speculum examination may reveal an open external cervical os with a fleshy-type endocervical mass presenting. With early transvaginal ultrasound, these implantations are easily identified (Fig. 25.5) and can, thus, be treated with conservative fertility-sparing options.
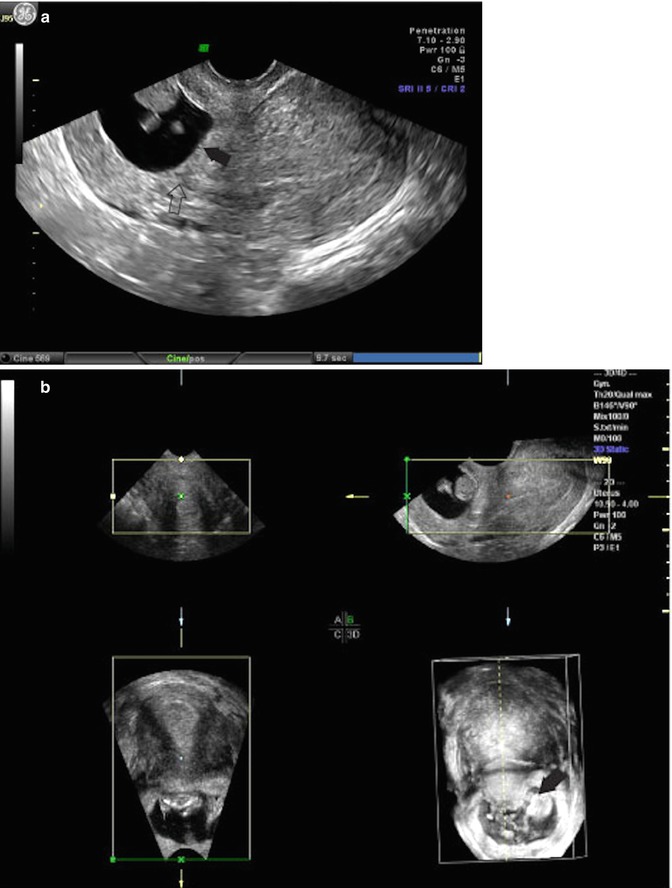

Fig. 25.5
(a) Transvaginal ultrasound, midline sagittal image: cervical pregnancy, (closed arrow) points to the cervical pregnancy within the (open arrow) points to cervical canal. (b) Transvaginal ultrasound: 3D rendering of a cervical pregnancy, (closed arrow) points to the internal cervical os
Rankin suggested that the diagnosis by ultrasound examination of cervical pregnancy required 4 criteria: enlargement of the cervix, uterine enlargement, diffuse amorphous intrauterine echoes, and absence of an intrauterine pregnancy [17]. Timor-Tritsch et al. refined the criteria to include the placenta and entire chorionic sac containing the pregnancy must be below the internal cervical os and the cervical canal must be dilated and barrel shape [18].
If necessary to exclude the diagnosis of a spontaneous abortion in progress, the presence of embryonic cardiac activity and/or Doppler ultrasound indicating vascular attachment confirms a living pregnancy.
Ovarian Pregnancy
One half of one percent to almost 3 % of ectopics are implanted within the ovary [11, 19]. Ovarian pregnancy like other non-tubal ectopic pregnancies may occur without the usual expected antecedent risk factors for ectopic pregnancy but does seem to have a strong association with conceptions with an intrauterine contraceptive device in place [20, 21]. The presenting signs and symptoms are similar to other ectopic pregnancies: positive pregnancy test, abdominal pain, and vaginal bleeding.
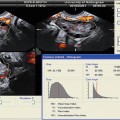
It is difficult to preoperatively make the diagnosis of ovarian pregnancy. An ultrasound finding suggesting ovarian implantation is a walled cystic mass within or adjacent to an ovary, but this does not exclude a corpus luteum and a tubal implantation. Doppler cannot distinguish between a corpus luteum and an ovarian pregnancy implantation (Fig. 25.6). This diagnosis is usually a pathological diagnosis: made by microscopic examination of a surgically removed adnexal mass, via laparotomy or laparoscopy, based on Speigelberg’s criteria: the tube must be intact and distinctly separate from the ovary, the gestational sac must occupy the normal anatomical location of the ovary, the gestational sac must be connected to the uterus by the utero-ovarian ligament, and unquestioned ovarian tissue must be demonstrated in the wall of the gestational sac [22].
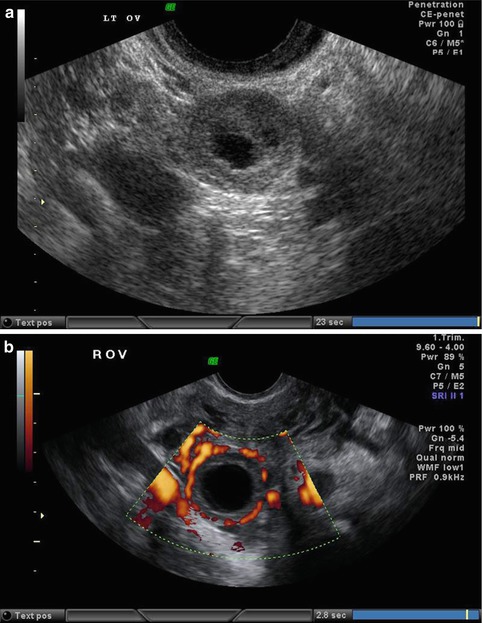

Fig. 25.6
(a) Transvaginal ultrasound of an ovarian corpus luteum cyst. (b) Transvaginal ultrasound: color Doppler imaging of an ovarian corpus luteum cyst
It is important for the laparoscopic surgeon to understand that an ovarian pregnancy can look like a corpus luteum ovarian cyst upon direct inspection, and cystectomy and pathology only will reveal the true diagnosis. However, when an adnexal ectopic is diagnosed with a nonsurgical algorithm, conservative medical therapy can be successful without a true diagnosis of location.
Abdominal Pregnancy
Less than 1 % of ectopic pregnancies are implanted within the abdominal cavity [11, 23]. The pathogenesis of abdominal implantation is controversial. Many are the result of secondary nidation within the peritoneal cavity after tubal abortion, tubal rupture or uterine rupture [24]. True primary abdominal implantation must satisfy the criteria of Studdiford. Studdiford, reporting a primary peritoneal implantation in 1942, established three criteria for such a primary abdominal pregnancy: normal fallopian tubes with no evidence of recent or remote trauma, the absence of any uteroperitoneal fistula, and the presence of a pregnancy related exclusively to the peritoneal surface and early enough to eliminate the possibility of secondary implantation following a primary nidation within the tube [25].
The most common abdominal implantation site is the posterior cul-de-sac, followed by the mesosalpinx, the omentum, the bowel and its mesentery, and the peritoneum of the pelvic and abdominal walls, including the anterior cul-de-sac [23]. Other reported locations include the retroperitoneal space, the appendix, the liver, and the spleen [26–31].
With the universal use of early pregnancy imaging, the diagnosis can be confirmed at an early gestational age, but this requires imaging demonstrating a continuity of the cervix and uterus without pregnancy contents, like other ectopic implantations. The presence of an adnexal mass suggestive of ectopic pregnancy, when no intrauterine gestation is identified, could be an ectopic pregnancy of any location, including an abdominal implantation. Failure to follow basic ultrasound principles can miss the diagnosis. Such early diagnosis can spare maternal mortality at the expense of fetal mortality, with a perinatal mortality rate of 40–95 % [32].
Cesarean Scar Ectopic Pregnancy
Although previously rare, the incidence of pregnancy implantation within the scar of a prior cesarean is increasing due to the increasing number of cesarean deliveries. The natural history of such a condition is unknown, but uterine scar rupture and hemorrhage, even in the first trimester, seems likely if the pregnancy is allowed to continue, with possible serious maternal morbidity and the possible need for hysterectomy and loss of subsequent fertility. Early diagnosis of such implantation is made only with a high level of suspicion: early ultrasound in a woman with a prior cesarean delivery (Fig. 25.7).
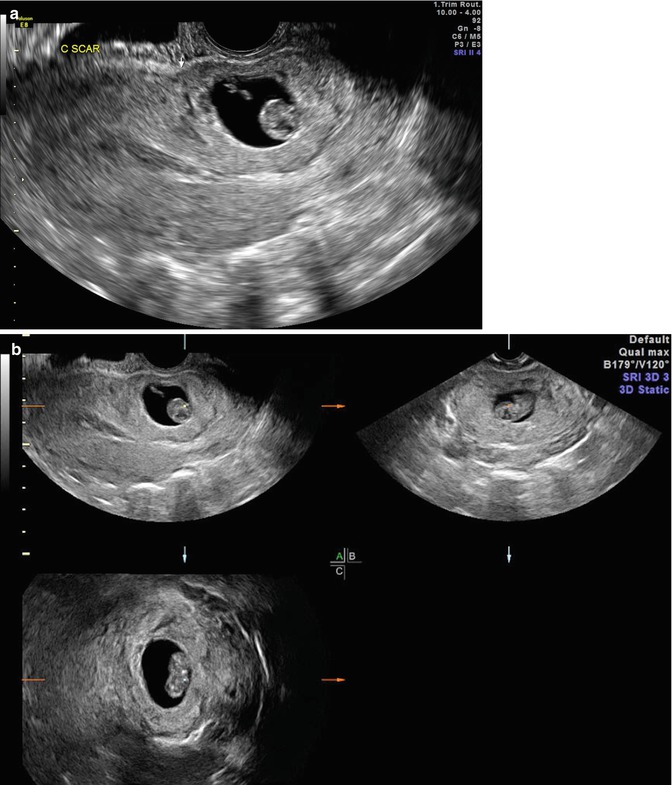

Fig. 25.7
(a) Transvaginal ultrasound: midline sagittal image with gestation in the anatomical location of a prior cesarean scar. (b) Transvaginal ultrasound: 3D rendering of cesarean scar ectopic
Endometrial and myometrial disruption or scarring can predispose to abnormal pregnancy implantation. Trophoblast adherence or invasion is enhanced when the scant decidualization of the lower uterine segment is impaired further by previous myometrial disruption. Implantation of a pregnancy within the uterine scar of a prior cesarean delivery is different from an intrauterine pregnancy with placenta accreta. Cesarean scar implantation is a gestation completely surrounded by myometrium and the fibrous tissue of the scar and separated from the endometrial cavity or fallopian tube (Fig. 25.7). The mechanism that most probably explains scar implantation, like intramural implantation, is invasion of the myometrium through a microscopic tract. Like intramural pregnancy, such a tract is believed to develop from the trauma of previous uterine surgery, such as curettage, cesarean delivery, myomectomy, metroplasty, hysteroscopy, and even manual removal of the placenta [33–35]. The time interval between such trauma and a subsequent pregnancy may impact upon implantation events. Some of the reported cases were diagnosed and treated within a few months of a prior cesarean delivery suggesting that incomplete healing of the uterine scar may contribute to scar implantation [36




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree