Summary of Key Points
- •
Normal fallopian tubes are difficult to visualize with ultrasound unless surrounded by small amounts of free peritoneal fluid.
- •
Classic sonographic signs that help distinguish a dilated fallopian tube from other cystic adnexal masses include waist, incomplete septation, cogwheel, and beads on a string signs.
- •
Acute pyosalpinx is diagnosed on ultrasound examination by the finding of a dilated, tubular adnexal structure filled with complex fluid and a thick, hyperemic wall.
- •
A tubo-ovarian abscess (TOA) typically presents as a complex adnexal mass with solid and cystic components, thick walls, hyperemia, and an indistinguishable ovary.
- •
Hematosalpinx secondary to endometriosis appears on ultrasound images as a dilated tubular adnexal structure filled with fine low level echoes.
- •
Isolated tube torsion is a rare event, often presenting as a dilated fluid-filled tubular structure with a V-shaped configuration or beak-like pointed narrowing at the twisted edge of the tube and is often found in an unusual location.
- •
The classic clinical presentation of primary fallopian tube malignancy consists of intermittent bloody vaginal discharge, colicky pain relieved by discharge, and a pelvic mass.
- •
Tube patency can be evaluated sonographically using agitated saline or ultrasound contrast agents.
Unlike the uterus and ovaries, the normal fallopian tube is usually not visible on ultrasound images, and, therefore, documentation of the fallopian tube is not a standard requirement for pelvic sonography. Indeed, when visualized, the fallopian tube is usually dilated or thickened and hence abnormal. Further confounding the evaluation of the fallopian tubes is the relative difficulty in distinguishing an abnormal fallopian tube from a complex cystic ovarian mass. The goals of this chapter are to describe the appearance of the normal fallopian tube; review the pathologic processes that result in abnormalities of the tube, both benign and malignant and either acute or chronic; and demonstrate a variety of sonographic findings that can aid the sonographer in correctly distinguishing tube versus ovarian origin of an adnexal mass.
Embryology and Normal Anatomy
The fallopian tube serves to connect the ovary to the uterus, allowing passage of the fertilized oocyte into the endometrial cavity. Embryologically, the paired paramesonephric ducts develop from coelomic epithelium during the 5th to 6th weeks of gestation. As the caudal portions fuse to form the uterus, the cranial aspects become the fallopian tubes with the funnel-shaped end remaining open to the peritoneum. The normal fallopian tubes are 10 to 12 cm long and between 1 to 4 mm in diameter and are located in the mesosalpinx, a fold of the peritoneum within the broad ligament.
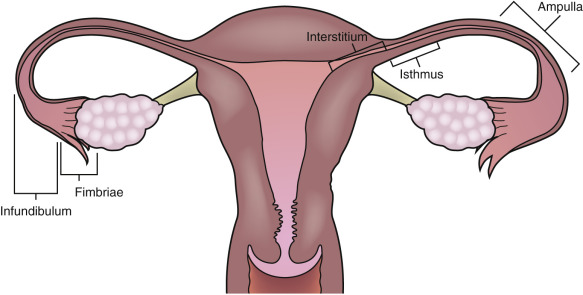
The fallopian tube is divided into four anatomic components ( Fig. 31-1 ). The interstitial segment is intramural and found within the uterine cornua near the fundus. The interstitial segment courses from the serosal surface of the uterus through the myometrial wall to open into the endometrial cavity. It is the shortest (2 cm) and narrowest (1 mm) portion of the fallopian tube. The isthmic segment is closest to the uterus with a length of approximately 3.5 cm and a diameter of 2 mm. The ampulla of the tube is the most lateral portion of the fallopian tube as well as the widest and longest section measuring 6 to 7.5 cm in length. The ampulla terminates at the funnel-shaped infundibulum, which has a fimbriated end that drapes over the ovary. Histologically, the plicae or folds of the fallopian tube are more numerous and complex in the ampulla and infundibulum where the ciliated columnar epithelial cells are also more numerous. Peg cells located between the ciliated cells produce the tubular fluid, which provides nutrients for the spermatozoa and oocyte. This fluid progresses toward the lateral end of the fallopian tube near the ovary in the opposite direction of the motion of the cilia and is excreted into the peritoneal cavity, accounting for the frequent finding of a small amount of simple free fluid in the female pelvis at all stages of the menstrual cycle. The fallopian tube functions to capture the released ovum from the ruptured ovarian follicle and provides a hospitable environment for fertilization and subsequent transfer of the fertilized ovum to the uterus as it grows and differentiates into the blastocyte. The ovum is propelled through the tube toward the uterus by the ciliated epithelium and mucosal plicae as well as muscular contractions in the wall of the tube.
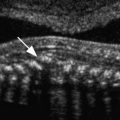
Normal fallopian tubes can occasionally be visualized with transvaginal sonography, especially when there is some fluid in the adnexal regions and minimal pressure is exerted on the probe. The normal fallopian tube appears solid, separate from the ovary and isoechoic to the uterus ( Fig. 31-2 ). The presence of a paratubal cyst, also known as a hydatid of Morgagni, may help localize the tube. These paratubal cysts are usually müllerian remnants of the paramesonephric duct. Most often, they are small, unilocular, simple cysts located at the fimbriated end of the fallopian tube between the tube and the ovary ( ). An echogenic fat plane between the cyst and the ovary as well as absence of a surrounding rim of ovarian parenchyma serve to differentiate a paratubal cyst from an exophytic ovarian cyst ( Fig. 31-3 ). Simple paratubal cysts are considered a benign finding, and the vast majority of paratubal cysts are of no clinical significance. However, a large paratubal cyst can rupture or serve as a lead point for isolated tube torsion. Rarely, papillary projections in a large (>5 cm) paratubal cyst will indicate malignancy, usually a borderline tumor. Other congenital anomalies of the fallopian tube include hypoplasia, aplasia, ectopic location, and herniation (with the ovary) and are much less common.

Sonographic Signs of Abnormal Fallopian Tubes
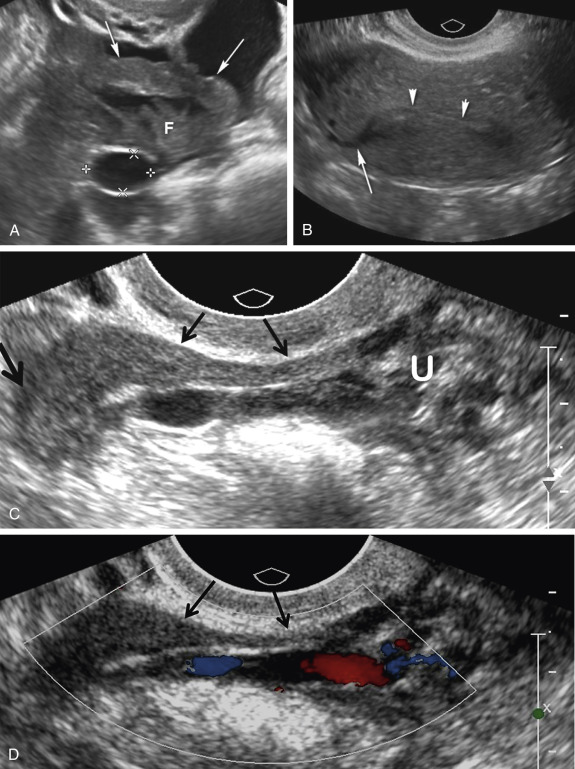
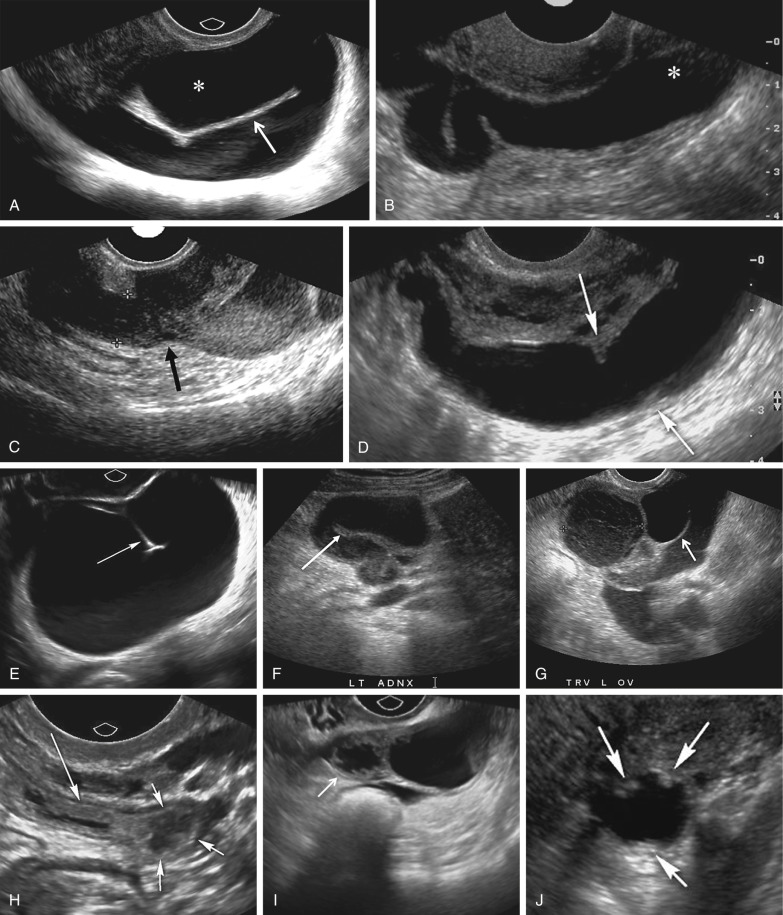
A variety of sonographic characteristics and signs have been described to help distinguish a dilated fallopian tube from an ovarian mass or other tubular structures in the pelvis such as bowel loops, hydroureter, and varices. Peristalsis, connection to other loops of bowel, as well as the classic striated echopattern or “gut signature” of the bowel wall will serve to distinguish a fluid-filled loop of bowel from a dilated fallopian tube. A dilated ureter can often be traced to the ureterovesicular junction (UVJ) on transabdominal or transvaginal sonography, and color Doppler should always be used to evaluate any apparent fluid-filled or cystic structure to exclude vascular pathology such as ateriovenous malformations or pelvic varices ( Fig. 31-4 ). Asking the patient to perform a Valsalva maneuver may increase flow in a pelvic varix, making it easier to confirm on color Doppler imaging. Identification of the ovary that is clearly separate from an adnexal mass is the single most helpful finding in distinguishing an abnormal or dilated fallopian tube from a complex cystic ovarian lesion. As the fallopian tube dilates, it often develops an S, U, V, C, or serpiginous shape ( Fig. 31-5A and B ). The ampullary segment is often wider than the isthmus, and an abrupt transition in diameter between the two segments is often observed ( Fig. 31-5C ). A waist sign, consisting of diametrically opposed indentations in the wall ( Fig. 31-5D ), may be observed, usually at the junction of the ampullary portion of the tube with the much wider fimbria. If the dilated tube folds back on itself, the juxtaposition of the two inner walls will create the incomplete septation sign ( Fig. 31-5A, E, F, and G ), consisting of a linear echogenic protrusion arising from one wall but not reaching the opposite wall. Both cine clips and two-dimensional and three-dimensional reformats can help display the serpiginous nature of the dilated tube ( ). When the thickened plicae or endosalpingeal folds are visible projecting into the lumen, one may observe numerous 2- to 3-mm echogenic mural-based masses projecting into the lumen of the dilated tube, creating the cogwheel sign ( Fig. 31-5H and I ) or the beads on a string sign ( Fig. 31-5J ) as tube markers. These signs are more frequently identified when the tube is only mildly dilated. The wall of the tube is thicker with the cogwheel sign and thinner with the beads on a string sign, with the latter more likely to be seen with chronic inflammation. As the tube becomes increasingly dilated, the thickened plicae are effaced and the hydrosalpinx assumes a more nonspecific cystic appearance with a thin, regular wall. The combination of a tubular shape, waist sign, and visualization of the ovary separate from an adnexal mass has been reported to be the most specific constellation of ultrasound findings to differentiate a dilated fallopian tube from a complex cystic mass of ovarian origin. It should be appreciated that visualization of a hydrosalpinx indicates that the tube is blocked, most often secondary to adhesions.

Pelvic Inflammatory Disease
Pelvic inflammatory disease (PID) is usually caused by sexually transmitted organisms that ascend from the vagina and cervix to the upper female genital tract. Approximately 770,000 acute cases are reported yearly in the United States. The number of subacute and chronic cases is unknown. Risk factors for PID include young age, multiple sexual partners, lack of barrier contraception, low socioeconomic class, and smoking. The inciting organisms are usually Chlamydia trachomatis and Neisseria gonorrhoeae , which cause epithelial damage to the tubes, allowing superinfection with polymicrobial opportunistic organisms. The infection initially ascends from the cervix to involve the endometrium causing endometritis and progresses to involve the fallopian tubes resulting in tube inflammation. Tube adhesions may form causing obstruction leading to hydrosalpinx, hematosalpinx, or pyosalpinx. Eventually the infection will spread to the ovary and peritoneum causing uterine serositis, peritonitis, inflammation of the pelvic fat, tubo-ovarian complex (TOC), and finally tubo-ovarian abscess (TOA). Symptoms include pelvic pain, cervical motion tenderness, purulent vaginal discharge (which may be foul smelling), fever, leukocytosis, and elevated erythrocyte sedimentation rate or C-reactive protein level. The degree of pelvic pain is highly variable. Inflammation around the liver can result in right upper quadrant (RUQ) pain, termed Fitz-Hugh–Curtis syndrome. Complications following PID include pelvic abscess, infertility (20%), ectopic pregnancy (9%), and chronic pelvic pain (18%).
Because symptoms are insufficient for diagnosis in up to 50% of cases and often overlap with other pelvic conditions such as appendicitis, ruptured or hemorrhagic ovarian cysts, and diverticulitis, imaging is frequently performed. In addition, imaging may be required if the patient is not responding appropriately to antibiotic therapy, particularly if an abscess is suspected. Sonography should be the first imaging study obtained, although occasionally computed tomography (CT) or magnetic resonance imaging (MRI) may be required to assess the full extent of an abscess, especially when rupture is considered likely. Despite the frequent use of ultrasound to assess patients with PID, there are no large trials evaluating the sensitivity and specificity of sonography for the diagnosis of PID. However, sonography likely has relatively low sensitivity for detecting mild abnormalities and is nonspecific for many others. When the fallopian tubes become involved, the specificity of sonography improves. Transvaginal sonographic imaging is considered most useful for detecting tube abnormalities and to identify the ovaries, with transabdominal imaging most often used to evaluate large pelvic abnormalities and to assess the full extent of ascites.
Cervicitis is typically occult on ultrasound examination and is diagnosed by direct visualization and culture. Endometritis will appear on ultrasound imaging as indistinct thickening of the endometrium, which is often extremely vascular. The endometrial cavity may be distended with fluid, hemorrhage, and even air, which is characterized on sonography by the presence of echogenic foci with dirty posterior shadowing. However, there is overlap in the sonographic appearance as well as the clinical presentation of endometritis with normal postpartum changes and retained products of conception. In addition, endometritis may develop postpartum or in patients with retained products of conception.
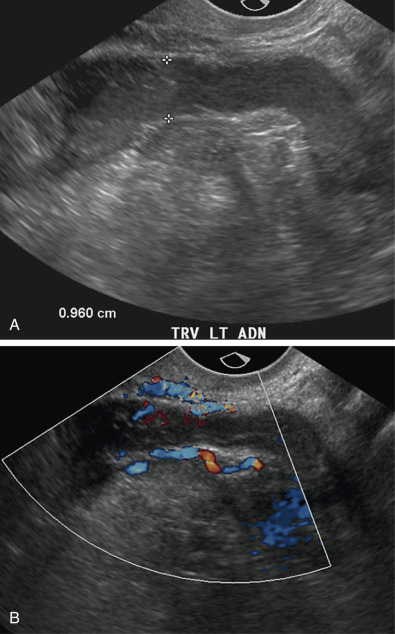
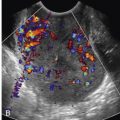
Involvement of the fallopian tube or ovary is the hallmark of PID and results in a variety of sonographic findings. Salpingitis manifests as mild thickening and hyperemia of the tube ( Fig. 31-6 ) and peritubal inflammation. Sonographically, these changes result in a prominent or thick tube that is thus more easily visualized, and hyperemia is typically observed on color Doppler imaging. Increased echogenicity and vascularity in the surrounding pelvic fat is common as a result of inflammation or infection ( Fig. 31-7 ). Small amounts of adjacent complex free fluid may be present and the ovaries may appear enlarged and edematous with an appearance simulating polycystic ovary disease.