Outline
Normal and Inconsequential Findings, 920
Ovarian Cysts With Typical Benign Features, 920
Ovarian Cysts With Typical Malignant Features, 924
Ovarian Cysts With Indeterminate Features, 925
Solid Ovarian Masses, 926
Pitfalls in Evaluating the Ovary, 928
Ovarian Masses in Pregnancy, 929
Syndromes and Diseases Associated With Ovarian Neoplasms, 929
Summary, 930
Summary of Key Points
- •
A simple cyst less than 3 cm in the ovary of a premenopausal patient is best termed a follicle and is a normal finding.
- •
A simple cyst less than 1 cm in the ovary of a postmenopausal patient is considered inconsequential.
- •
Most ovarian masses are benign and have a typical sonographic appearance that allows accurate diagnosis.
- •
Many simple and hemorrhagic cysts do not need sonographic follow-up in asymptomatic patients.
- •
Before diagnosing a simple ovarian cyst, it is important to search carefully for small nodules along the wall.
- •
A solid area with flow on Doppler imaging is the most important morphologic characteristic of an ovarian mass that raises concern for malignancy.
- •
The occasional indeterminate appearing ovarian mass can be managed variably by repeat sonography, magnetic resonance imaging (MRI), or surgical evaluation.
Pelvic sonography, including transvaginal scanning, is the preferred initial imaging modality for evaluation of a suspected ovarian or other adnexal mass. Its high sensitivity and specificity for ovarian malignancy, lack of ionizing radiation, relatively low cost, and wide availability make it an ideal method for evaluation of the ovary. In most patients, sonography is adequate to evaluate an ovarian mass. Scoring systems have been used to characterize ovarian and other adnexal masses sonographically, and they perform reasonably well. However, subjective assessment has been shown to perform as well or better than mathematical scoring systems. Although accurate and timely identification of ovarian malignancy is extremely important, most adnexal masses are benign and most have a typical sonographic appearance. Thus, it is essential to recognize these common benign ovarian masses as frequently as possible and not mistake them for ovarian malignancy. Appropriate sonographic characterization of adnexal masses may help prevent unnecessary follow-up imaging along with its attendant patient anxiety and unnecessary surgery as well as its attendant risks. Detailed sonographic evaluation can prompt referral to gynecologic oncologists for management of adnexal masses that are likely to be malignant. When an adnexal mass has one of the classic benign appearances (to be discussed in this chapter), characterization is complete, although some may warrant sonographic follow-up. If a mass has characteristic malignant findings, imaging for characterization or diagnosis is also typically complete, though further evaluation for staging may be needed. For masses with indeterminate sonographic findings, management will vary depending upon the clinical circumstances, but options would typically include follow-up sonography, MRI, or surgical evaluation. Standardized terminology and reporting have been suggested for ovarian masses; neither has been widely adopted at this time, but both may be further developed in the future.
In a small minority of patients, additional pelvic imaging with MRI may be helpful when ultrasound fails to clarify the origin of an adnexal mass, when the sonographic features are indeterminate, or when an adnexal mass is inadequately imaged with ultrasound (such as in an obese patient or in one who cannot undergo or declines transvaginal scanning). Although computed tomography (CT) is helpful in staging of patients with known or suspected ovarian malignancy, it does not usually have a significant role in the characterization of adnexal masses. CT may occasionally be helpful if a gastrointestinal origin of an adnexal mass is suspected or to search for a primary neoplasm when ovarian metastases are suspected. Positron emission tomography/CT has little, if any, role at this time in the primary evaluation of ovarian masses, although it too may be helpful to search for a primary neoplasm if ovarian metastases are suspected.
In this chapter, we will review a few normal findings specific to the ovary, discuss sonographic features of benign and malignant ovarian masses, present an approach to assessing indeterminate ovarian masses, and review pitfalls in evaluation of the ovary. When appropriate, the findings and recommendations of the Society of Radiologists in Ultrasound consensus conference on ovarian and other adnexal cysts have been included in this chapter. Some aspects of ovarian disease will not be discussed or will be mentioned only briefly, as they are covered elsewhere in this text. Ovarian torsion is discussed in Chapter 29 , nonovarian adnexal masses including tubo-ovarian abscess in Chapter 31 , and polycystic ovary syndrome and ovarian hyperstimulation syndrome in Chapter 32 .
Normal and Inconsequential Findings
Although normal ovarian findings and ultrasound technique is more thoroughly discussed in Chapter 26 , a few observations unique to the ovary bear additional mention here ( Fig. 30-1 ). Ovarian follicles typically achieve a size of 2 to 3 cm before ovulation. Hence, simple (unilocular, thin-walled, anechoic) ovarian cysts less than 3 cm in greatest diameter in premenopausal women should generally be considered normal findings. In order to prevent confusion with pathologic findings, it is best not to use the term “cyst” for normal ovarian structures and better to describe them as follicles or to simply report the ovary as normal. Simple ovarian cysts less than 1 cm in greatest diameter may be present in postmenopausal women and have been reported in approximately 20% of women 5 or more years following menopause. Hence, simple ovarian cysts less than 1 cm in maximal diameter in a postmenopausal woman generally require no follow-up and are considered inconsequential. The corpus luteum, which typically appears as a thick-walled cystic lesion less than 3 cm in diameter with internal echoes and crenulated wall, should also be recognized as a normal finding in premenopausal women. The wall of the corpus luteum may be quite vascular on Doppler interrogation.

Echogenic foci are a normal finding in many ovaries ( Fig. 30-2 ). Tiny echogenic foci, measuring 1 to 3 mm in width, have no posterior shadowing (although comet-tail artifact may be seen) and may be due to psammomatous calcifications associated with epithelial inclusion cysts, hemosiderin deposition, or bright specular reflections off the back walls of tiny follicles. These ovarian echogenic foci are found both in premenopausal and postmenopausal women. They are generally of no significance and may occasionally be helpful in identifying an ovary. Larger echogenic foci in the ovary, usually from isolated calcifications, are also typically benign findings ( Fig. 30-3 ). Echogenic foci 5 mm and larger, some of which may demonstrate posterior acoustic shadowing, seen in otherwise normal ovaries have been attributed to corpus albicans. Some association between these foci and adenofibromas has been reported. The pattern of calcifications within the ovary should be examined, as a more extensive peripheral rind of calcifications has been reported in a patient with endosalpingiosis and serous borderline ovarian neoplasms. Larger coarse ovarian calcifications, in the absence of a mass, are generally benign and can be followed sonographically. Anecdotal evidence suggests that patients with larger or more extensive calcifications in an otherwise normal-appearing ovary should receive additional evaluation or closer follow-up.


Ovarian Cysts with Typical Benign Features
Ovarian lesions with classic features of simple cysts, hemorrhagic cysts, endometriomas, or dermoids are highly likely to be benign. It is important to recognize the characteristic sonographic features that, when seen, are highly predictive of these benign entities. Reliable characterization of ovarian masses using these sonographic features requires that the mass be fully and adequately visualized by ultrasound. Occasionally, in a patient with a suboptimal ultrasound examination, the interpreting physician will need to decide how to proceed based on available imaging findings and degree of clinical concern. When reporting on adnexal masses seen on pelvic sonograms, it is important to describe specific sonographic features (detailed later) that allow one to determine the likely diagnosis. Use of the word “complex” as a descriptor, with no additional explanation, is problematic as the “catch-all” term is often used for any cystic mass that is not a simple cyst. There are many features that may result in a “complex” sonographic appearance, including a typical reticular pattern suggesting benign hemorrhagic cyst and typical solid nodule worrisome for malignant ovarian carcinoma. Thus, if an ovarian cyst is reported as “complex,” further descriptors detailing those features making it complex should also be provided.
Simple Cysts
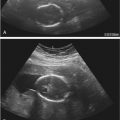
As with cysts elsewhere in the body, ovarian cysts with thin walls, anechoic internal contents, posterior acoustic enhancement, and no septations or solid components meet sonographic criteria for simple cysts ( Fig. 30-4 ). Follicular or corpus luteal cysts and serous cystadenomas may appear as simple cysts by sonographic criteria. Simple ovarian cysts occur in 4% to 17% of postmenopausal women and the majority resolve or remain stable on follow-up ultrasound evaluation. However, annual follow-up sonography for simple ovarian cysts larger than 1 cm (though some practices may choose to raise this threshold to 3 cm) is recommended in postmenopausal women. In premenopausal women, it is recommended that cysts between 5 and 7 cm in largest diameter be followed yearly by ultrasound examination. The vast majority of simple ovarian cysts are benign. With increasing cyst size, however, there is a risk of inadequate assessment of the cyst wall for detection of small solid nodules or papillary formations, which, if present, increase the likelihood of malignancy. The rare occurrence of malignancy in an apparent simple ovarian cyst is more likely in larger cysts, in which small mural nodules may be overlooked. Hence, when evaluating what appears to be a simple cyst, it is important to confirm that the cyst is adequately imaged in its entirety and to carefully assess for small nodules before concluding that it is indeed a simple cyst. Simple cysts measuring larger than 7 cm are still likely benign, though one should consider further imaging evaluation with MRI to confirm that there is no solid component overlooked by sonography.

Hemorrhagic Cysts
Fibrin strands and retracting clot are highly specific features of hemorrhagic ovarian cysts ( Fig. 30-5 ). The fibrin strands are often described as lacy, reticular, fishnet, cobweb, spider web, or sponge-like in appearance. Although retractile clot might be confused with a solid mural nodule, it will have no detectable flow by Doppler imaging and typically has scalloped, concave, or straight margins. These features can help differentiate retracting subacute clot from malignant solid tissue, which is usually more round or lobular in configuration and often has demonstrable flow on targeted Doppler imaging. Cystic ovarian lesions without detectable internal flow on Doppler imaging and with either the fibrin strand or retractile clot pattern of internal echoes very likely represent hemorrhagic cysts, and typically resolve within 8 weeks. Such sonographically typical hemorrhagic cysts less than 5 cm in maximal diameter do not generally need follow-up sonography in asymptomatic premenopausal patients. However, sonographically typical hemorrhagic cysts larger than 5 cm, suspected hemorrhagic cysts with atypical morphologic features, and hemorrhagic cysts in perimenopausal or early postmenopausal (1 to 5 years after final menstrual period) women should be reevaluated by follow-up ultrasound evaluation in 6 to 12 weeks to ensure resolution or decrease in size. Women in late postmenopause (greater than 5 years since final menstrual period) would not be expected to develop hemorrhagic ovarian cysts. It is unlikely that one would suspect a hemorrhagic ovarian cyst based on ultrasound criteria in late postmenopause, but if this were to occur, one should consider the possibility of neoplasm and recommend further evaluation with MRI or surgical consultation.

Hemorrhagic ovarian cysts may contain solid-appearing areas due to clot with concave or straight margins and lack of detectable flow by careful Doppler interrogation. There are occasional problematic cases in which apparently solid areas without detectable flow have outwardly convex margins and thus may simulate solid mural neoplastic nodules. Gentle pressure on the cyst with the transvaginal transducer is sometimes helpful, as an intracyst clot may show jiggling or jelly-like motion with this maneuver. If the diagnosis remains uncertain, follow-up ultrasound imaging is often helpful in distinguishing a hemorrhagic cyst (with interval resolution of clot) from an ovarian neoplasm (with persistence, enlargement, or apparent development of internal blood flow). Occasionally a hemorrhagic cyst may simulate a solid lesion with a sonographic appearance of diffuse heterogeneous internal echoes ( Fig. 30-6 ), typically encountered in an acute or subacute stage, before fibrin strands develop. Lack of detectable flow by Doppler imaging (using settings optimized to detect low-volume, low-velocity flow) within the apparently solid component, posterior acoustic enhancement, and awareness of this entity in premenopausal women can suggest this possibility. Resolution on follow-up ultrasound examination would confirm the diagnosis of self-limiting hemorrhagic cyst.

The most significant potential complication of a hemorrhagic ovarian cyst is rupture with hemoperitoneum. A ruptured hemorrhagic cyst may be difficult to distinguish from a ruptured ectopic pregnancy, thus necessitating correlation with serum human chorionic gonadotropin (hCG) level. A ruptured ectopic pregnancy typically requires operative management, whereas a ruptured hemorrhagic ovarian cyst can usually be managed expectantly in hemodynamically stable patients.
Endometriomas
Endometriosis occurs when there is endometrial tissue outside the uterus. Most cases are reported in women of childbearing age and a 10-fold increase in prevalence has been reported in patients with an affected first-degree relative. Although some may be asymptomatic, patients most commonly present with pain, which may be cyclic, correlating with their menstrual cycle, or infertility. Some patients with endometriosis will form cysts, termed endometriomas, which are found in up to 44% of women with endometriosis. Most endometriomas are located within the ovary. The pathogenesis of intraovarian endometriomas may be different than that of endometriosis that occurs as superficial peritoneal implants. Endometriomas are usually readily detectable by ultrasound. Cystic lesions with diffuse low-level internal echoes, sometimes described as “ground glass,” are characteristic, with 95% of endometriomas having this appearance ( Fig. 30-7 ). Multilocularity and echogenic foci in the wall have been reported to increase the likelihood that a lesion represents an endometrioma ; however, neither is required for the diagnosis. In our experience, it is uncommon for endometriomas to be multilocular though there may be multiple adjacent endometriomas that could be difficult to distinguish from multilocularity. Lack of acoustic streaming (movement of echoes inside the cyst during gray-scale or Doppler ultrasound imaging) was initially thought to be predictive of an endometrioma, but subsequent studies have not found absence of acoustic streaming to be reliable for this diagnosis.

Surgical removal is considered for symptomatic endometriomas, for those causing pain and infertility, as well as for those in which malignancy is suspected. Fertility does not always improve following cystectomy, however, as surgery can reduce the number of viable ovarian follicles. Malignancy, typically clear cell carcinoma and less often endometrioid adenocarcinoma, should be suspected in endometriomas that develop solid mural nodules or that rapidly increase in size. MRI can be helpful in assessing endometriomas for enhancing mural nodules and for restricted diffusion in those suspected of undergoing malignant transformation. Asymptomatic lesions with typical sonographic appearance of endometriomas can be followed annually with ultrasound.
Given that the sonographic appearance of endometriomas and hemorrhagic cysts occasionally overlap, follow-up sonogram, typically in 6 to 12 weeks, is suggested, particularly if surgical removal of a presumed endometrioma is planned. If the lesion is a hemorrhagic cyst, it will resolve or change on follow-up study. Endometriomas in postmenopausal women may differ in appearance from the typical homogeneous echotexture seen in premenopausal women and may be more heterogeneous with echogenic foci centrally. A small number of endometriomas may contain a small solid-appearing area on ultrasound imaging, and, therefore, it can be difficult to distinguish these endometriomas from malignant lesions. Doppler sonographic imaging is suggested, although it may not resolve the diagnosis as the solid area may be due to focal endometrial tissue with internal blood flow. In such cases, additional evaluation with MRI should be considered.
Mature Cystic Teratomas
Mature cystic teratomas of the ovary, also termed dermoids, account for up to 20% of ovarian neoplasms. These benign germ cell tumors are composed of at least two of the three germ cell layers (ectoderm, mesoderm, and endoderm). Dermoids are estimated to account for up to 20% of all ovarian tumors found in adult women and are bilateral in 15% to 25% of cases. Most dermoids are asymptomatic and are incidentally detected. However, dermoids may present with symptoms related to large size resulting in compression of adjacent structures. Torsion or rupture of a dermoid may cause significant pain. Several characteristic sonographic appearances have been described, including focal or diffuse hyperechoic component; areas of acoustic shadowing, also known as the “tip of the iceberg” sign; and echogenic lines and dots, also referred to as dermoid “mesh” or “dot-dash” sign. Any combination of these classic sonographic features allows a confident diagnosis of a dermoid ( Fig. 30-8 ). The hyperechoic component, termed a Rokitansky nodule, typically corresponds to mixed hair and sebaceous material or occasionally to calcification, sometimes related to a bone or tooth. The echogenic lines and dots represent hair in fluid. Fluid-fluid levels may occur in dermoids but are seen infrequently. A dermoid can be confidently suggested if the nondependent fluid is hyperechoic (indicating fat). However, teratomas can display nondependent hypoechoic fluid and thus be difficult to distinguish from other cystic lesions with fluid-fluid levels. In such cases, it is important to look for other sonographic features suggesting a dermoid. Floating echogenic globules within a large mass is an uncommon appearance but is reported to be highly predictive of a dermoid. Calcifications can occur in dermoids, but calcification alone is not enough to make the definitive diagnosis.

Dermoids can increase in size but tend to do so slowly, with a mean growth rate of 1.8 mm per year in premenopausal women. A serious potential complication of mature cystic teratomas is malignancy—occurring in up to 2% of teratomas, 80% of which are squamous cell carcinoma. Additional potential complications include hyperthyroidism resulting from teratomas containing a large amount of thyroid tissue ; chemical peritonitis following spontaneous or iatrogenic dermoid rupture ; and ovarian torsion. Torsion has been reported in 3.5% of dermoids and is more common with larger lesions. Given the possibility of malignant transformation and of interval growth, which could increase the risk of ovarian torsion, annual sonographic follow-up of dermoids should be considered. The reliability of ultrasound in identifying malignant transformation of dermoids is not well established. Features suggestive of malignant transformation include isoechoic branching structures, demonstration of central flow within the mass by Doppler imaging, or findings of metastatic disease.
Ovarian Cysts with Typical Malignant Features
Ovarian neoplasms, both benign and malignant, are usually classified into one of four general histologic groups: epithelial, sex cord–stromal, germ cell, or metastatic neoplasms. There are also borderline epithelial neoplasms that are usually considered malignant, though they confer a better prognosis than frankly malignant lesions, even if peritoneal spread is noted at the time of diagnosis. Borderline tumors tend to occur in younger women. Most ovarian malignancies are epithelial neoplasms and demonstrate a mixture of cystic and solid components. Epithelial neoplasms include several histologic types, including serous, mucinous, endometrioid, and clear cell cystadenocarcinomas. The serous and mucinous forms also have benign counterparts, namely serous and mucinous cystadenomas. There is recent evidence that some epithelial ovarian carcinomas actually arise from the fallopian tube. Most ovarian cystadenomas do not undergo malignant degeneration into carcinoma or, if transformation does occur, it is usually into a borderline neoplasm or low-grade malignancy and the rate of transformation is exceedingly slow. Recent studies suggest there may be two types of epithelial ovarian carcinomas. Type I tumors are low-grade neoplasms that present at an early stage with an indolent course and are believed to arise from precursor lesions in the ovary, such as cortical inclusion cysts lined by tubal epithelia (perhaps incorporated into the cyst during ovulation), serous cystadenomas, borderline neoplasms, or endometriosis. Type II tumors are highly aggressive malignancies that present with advanced stage at diagnosis and likely originate from precursors arising in the fimbriated segment of the fallopian tube epithelium. Current molecular and genetic research suggests that inactivating mutations of the tumor suppressor gene TP53 give rise to “p53 signatures” (short segments of fallopian tube epithelium that overexpress p53, a tumor suppressor protein) in the fimbriated portion of the fallopian tube, which may ultimately transform into serous tubal intraepithelial cancers (STICs). It is now believed that STICs are the likely precursors of all pelvic extrauterine high-grade serous carcinomas either in the fallopian tube or following implantation onto the ovary or peritoneal surfaces. TP53 mutations have been reported in over 90% of high-grade serous carcinomas and BRCA1/BRCA2 mutations (also tumor suppressor genes that help repair DNA damage) have been found in up to 50% of high-grade serous carcinomas.
Sex cord–stromal neoplasms tend to present as solid ovarian masses but can sometimes have a mixed cystic and solid appearance, especially when large. Germ cell neoplasms and metastatic neoplasms vary in sonographic appearance, some being solid and some mixed cystic and solid. Some specific ovarian neoplasms such as fibromas (the most common sex cord–stromal neoplasm), dysgerminomas (the most common malignant germ cell neoplasm), and many metastases, such as from breast carcinoma, are typically solid masses.
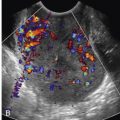
The presence of a solid component, with detectable flow by Doppler imaging, within a cystic ovarian mass is the most important sonographic feature for predicting ovarian malignancy. There is also evidence that the larger the solid component within a cystic mass, the higher the risk of malignancy. A small soft tissue component is more typical of borderline or stage I ovarian carcinoma than of advanced ovarian carcinoma. Apart from the characteristic hyperechoic tissue typical of dermoids, the presence of solid nodules (sometimes referred to as papillary projections, excrescences, or vegetations) or more confluent solid tissue with blood flow detected by Doppler imaging is highly likely to indicate malignancy ( Figs. 30-9 through 30-11 ). However, solid components are not a specific finding for ovarian malignancy, as solid mural nodules can be seen in benign cystadenomas and cystadenofibromas. The absence of a solid component makes ovarian malignancy unlikely. Focal wall thickening in a cystic mass is another sonographic feature worrisome for malignancy.
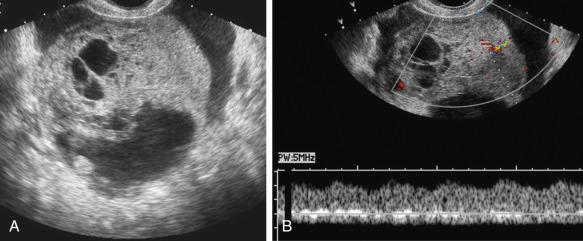


Irregular or thick (generally defined as >3 mm) septations are also concerning for malignancy, though they are less predictive than solid components. A cystic mass with thin septations and no solid component or a cystic mass with a solid component that has no detectable flow by Doppler imaging ( Figs. 30-12 and 30-13 ) is likely to be a benign ovarian neoplasm, such as a cystadenoma or cystadenofibroma. Additional findings of ascites (more than the trace physiologic amount common in premenopausal women), peritoneal implants ( Fig. 30-14 ), or evidence of metastasis are all worrisome findings suggesting malignancy but are not required to prompt suspicion of ovarian cancer.