Outline
Placental Development, 674
Normal Placenta, 676
Abnormalities of Placental Size and Shape, 677
Abnormalities of Placental Location, 678
Placental Cord Insertion Abnormalities, 681
Morbidly Adherent Placenta—Placenta Accreta, 683
Epidemiology and Risk Factors, 684
Sonographic Findings: Second and Third Trimesters, 684
Magnetic Resonance Imaging for Placenta Accreta, 684
First Trimester Placenta Accreta and Cesarean Scar Pregnancy, 686
Vascular Abnormalities of the Placenta, 687
Placental Sonolucencies, 687
Placental Calcification, 688
Placental Hematomas, 688
Placental Abruption, 689
Chorioangioma, 689
Placental Membrane Abnormalities, 691
Umbilical Cord, 693
Normal Development, 693
Remnants and Cysts, 694
Dimensions and Coiling, 695
Vascular Abnormalities of the Umbilical Cord, 695
Summary, 699
Summary of Key Points
- •
Assessment of the relationship between the placenta and the internal cervical os is an essential component of sonography in the second and third trimesters.
- •
Placenta previa is identified in 1% to 5% of second-trimester sonograms but is present at birth in only 3/1000 births.
- •
Transvaginal sonography is considered safe regardless of placental location, even in the presence of bleeding.
- •
Conditions associated with vasa previa include resolved placenta previa or low-lying placenta, velamentous cord insertion, and succenturiate or bilobate placenta.
- •
Sonographic criteria for detection of placenta accreta include placental lacunae, thinning of the retroplacental myometrium, and irregularity or disruption of the bladder-serosal interface, with bridging vessels between the placenta and the bladder-serosal interface on color Doppler imaging.
- •
A placental hematoma initially appears isoechoic to the placenta, becoming hypoechoic within 1 week and sonolucent in approximately 2 weeks.
- •
Targeted sonography is indicated if there are abnormalities of the umbilical cord or its vessels, including umbilical cord cyst, single umbilical artery, or persistent right umbilical vein, to evaluate for the presence of associated fetal anomalies.
Placental Development
In early human development, the blastocyst typically implants in the upper uterine corpus and contains two cell types. The outer trophoblast layer will form the placenta. The inner cell mass or embryoblast gives rise to the embryo, amnion, and umbilical cord ( Fig. 19-1 ). Following implantation, trophoblast cells at the embryoblast pole proliferate and become a double layer, each with its own function. The outer syncytiotrophoblast is a multinucleate syncytium that lacks distinct cells. It produces multiple hormones and is responsible for maternal-fetal gas, nutrient, and waste exchange. In contrast, the inner cytotrophoblasts have distinct cell membranes, are mitotically active, and serve as stem cells for the syncytiotrophoblast. During its life cycle, a cytotrophoblast first divides and proliferates. Then later, its cell walls disintegrate, and its contents merge into the expanding syncytiotrophoblast. At day 7 after conception, the syncytiotrophoblast at the embryoblast pole invades the decidua, which is endometrium that has been primed for pregnancy. Following initial invasion, the syncytiotrophoblast grows and spreads to surround the entire conceptus. During invasion, decidual blood vessels and glands are entered, and released blood fills lacunae or lakes within the syncytium ( Fig. 19-2 ). These lacunae eventually form the intervillous spaces. The syncytium is then invaded by cytotrophoblasts, and by definition, primary villi are thereby formed. Penetration extends the full thickness of the syncytiotrophoblast, and cytotrophoblasts meet at the bottom to form a circumferential trophoblast shell. Here, the primary villi are anchored.


Following cytotrophoblast invasion, extraembryonic mesenchyme moves into the primary villi to form secondary villi ( Fig. 19-3 ). Next, angiogenesis begins within secondary villi, which are then termed tertiary villi . During this same time, vasculature from the early umbilical cord fuses with vessels of the chorionic plate , that is, the fetal surface of the placenta ( Fig. 19-4 ). The basal plate is the opposite side, where the deeper invading trophoblast and the maternal decidua, specifically the decidua basalis, meet.


During the 7th week after menstruation, tertiary villi increase in diameter and develop into immature intermediate villi , which are important growth centers for villous tree branching. New branches begin first as syncytial sprouts. They are invaded by cytotrophoblasts and then by mesenchyme. Last, vessels develop within the mesenchyme. With this repeated branching and sprouting, the villous tree begins to attain its final form. Finally, at the beginning of the third trimester, slender mature intermediate villi branch to form terminal villi . During terminal villi angiogenesis, capillary growth exceeds villous growth. As a result, capillaries begin to coil and bulge to the periphery of a given villus. This bulging thins the trophoblast layers and creates the small diffusion distance needed for maternal-fetal exchange.
Distinct from these villous trophoblasts , some cytotrophoblasts break through the trophoblast perimeter and continue to invade into maternal tissue as extravillous cytotrophoblasts . These cells are further classified as interstitial trophoblasts and endovascular trophoblasts. The endovascular trophoblasts penetrate and plug maternal spiral arteries, which supply the decidua and intervillous space. Later, these cytotrophoblasts reverse their action and work to increase intervillous blood flow . For this, they invade the vascular media layer and replace its smooth muscle with fibrinoid material. This remodeling converts narrow-lumen, muscular spiral arteries into dilated, low-resistance uteroplacental vessels. In contrast, the interstitial trophoblasts invade the decidua and surround spiral arteries. Their functions are less well understood and may include vessel preparation for endovascular trophoblast remodeling.
Within the mature maternal-fetal circulation, oxygen-carrying maternal erythrocytes first enter the intervillous space via spiral arteries. Here, maternal blood is forced into close contact with terminal villi. Oxygen diffuses through the syncytiotrophoblast and then through the widely spaced but contiguous cytotrophoblast layer into fetal erythrocytes within capillaries of each terminal villous branch. Capillaries coalesce into larger fetal vessels within the main villus. From here, oxygenated fetal blood ultimately drains into the single umbilical vein and back to the fetus.
Normal Placenta
At term, the typical placenta weighs 470 g, is round to oval with a 22-cm diameter, and has a central thickness of 2.5 cm. It is composed of a placental disk, extraplacental membranes, and a three-vessel umbilical cord. The maternal surface is the basal plate , which is divided by clefts into portions—termed cotyledons. These clefts mark the site where internal septa originating from the decidua basalis push up into the intervillous space. The fetal surface is the chorionic plate , into which the umbilical cord inserts, typically in the center. Large fetal vessels that originate from the cord vessels then spread and branch across the chorionic plate before entering stem villi of the placental parenchyma. When examining the placenta, either following delivery or during fetoscopic surgery—for example, during laser therapy for twin-twin transfusion syndrome—the fetal arteries almost invariably cross over fetal veins. The chorionic plate and its vessels are covered by amnion.
Sonographically, the placenta in the first and early second trimesters appears homogeneous in echotexture and mildly hyperechoic compared with the underlying myometrium. It then becomes more isoechoic with advancing gestation ( Fig. 19-5 ). As examples, after midpregnancy, it is common to identify small placental sonolucencies, and in the third trimester, the placenta may appear more heterogeneous, with visible calcifications.

The sonographic thickness is usually greater than that of the gross specimen owing to collapse of intervillous spaces as blood drains from the placenta. As a general rule, the placental thickness in millimeters roughly approximates the gestational age in weeks. It does not normally exceed 4 cm in the second trimester or 6 cm in the third trimester. The retroplacental “clear” space normally measures less than 1 to 2 cm and, as the name suggests, appears hypoechoic. The retroplacental space is a common location of hematoma development. Inability to visualize the retroplacental space in a woman at risk for placenta accreta raises concern.
Abnormalities of Placental Shape and Thickness
Abnormal Placental Shape
In contrast to the normal architecture described previously, placentas may form as separate disks of nearly equal size. This bilobate or bilobed placenta is also known as placenta duplex. The cord inserts between the two placental lobes, either into a connecting chorionic bridge or into intervening membranes. Sonographic detection of a bilobate placenta that has a chorionic bridge—normal placental tissue connecting the two lobes—is not likely, particularly if not specifically sought. However, if placental lobes are separated by intervening membranes and the cord inserts directly into these membranes, such that the cord insertion is velamentous , then sonographic detection may help to avoid cord avulsion at delivery. A placenta containing three or more equal-sized lobes is rare and termed multilobate.
A succenturiate lobe is an accessory placental lobe that develops away from the main placental disk. The umbilical cord inserts into the main body of the placenta, and prominent vessels may be visible coursing along the intervening membranes ( Figs. 19-6 and 19-7 ). If an accessory placental lobe is suspected, color Doppler sonography may be particularly helpful in identifying the location and path of these vessels. Occasionally, the vessels that connect the main body of the placenta to a succenturiate lobe overlie the cervix, a form of vasa previa. Clinically, an accessory lobe may be retained in the uterus after delivery and cause postpartum uterine atony and hemorrhage. Succenturiate lobe is encountered more frequently with in vitro fertilization and twin gestations.


There are other rare placental shapes in which varying portions of the fetal membranes are covered by functioning villi. With placenta membranacea, all or nearly all of the membranes are covered with villi. This form of abnormal placentation may lead to serious hemorrhage, preterm delivery, and hysterectomy because of associated placenta previa or accreta. With ring-shaped placenta, the placenta is annular, and a partial or complete ring of placental tissue is present. With placenta fenestrata , the central portion of a placenta disk is missing. These variants are not typically visible sonographically.
Abnormal Placental Thickness
Placentomegaly , an abnormally thickened placenta, is diagnosed if the placental thickness exceeds 4 cm in the second trimester or 6 cm in the third trimester ( Fig. 19-8 ). There are numerous causes of placentomegaly, including maternal diabetes, severe maternal anemia, severe fetal growth restriction, aneuploidy, and congenital infections. The latter include syphilis, parvovirus infection, cytomegalovirus infection, toxoplasmosis, herpesvirus infection (rarely), and if at risk, rubella or schistosomiasis. Placentomegaly is a component of hydrops fetalis, and any cause that can result in immune or nonimmune hydrops may present with placentomegaly as a component. In some cases, placentomegaly may result from collections of blood or fibrin within the placenta. Examples include massive perivillous fibrin deposition, intervillous or subchorionic thromboses, and large retroplacental hematomas, which are described later. Neoplasia is a rare cause. Benign vascular lesions include chorioangioma and chorangiosis, also described in subsequent sections.

Gestational trophoblastic disease creates a thick cystic-appearing placenta. Cystic vesicles are also seen with placental mesenchymal dysplasia. Vesicles in this rare condition correspond to dilated stem villi that result from stromal expansion.
A placenta is not generally considered too thin, although focal attenuation may occur if a hematoma develops and subsequently resolves. A pregnancy with severe polyhydramnios may appear to have a thin placenta as a function of compression by fluid. A small placenta associated with a growth-restricted fetus may also appear thinner.
Abnormalities of Placental Location
The placental location may reliably be assessed by 16 weeks’ gestation. The location, whether anterior, posterior, left- or right-lateral, or a combination of these, should be noted in the sonogram report. A survey of the uterus for accessory placental tissue—a succenturiate lobe—and an assessment of the placental cord insertion site, if visible, should also be performed and documented. An evaluation of the relationship between the placenta and the internal cervical os is a component of the standard obstetric sonogram that is performed at approximately 18 to 20 weeks.
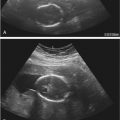
Transabdominal sonography is typically used to screen for abnormalities of placental location, and if the placenta is either clearly over the cervix or away from the lower uterine segment, its sensitivity and negative predictive value are excellent ( Fig. 19-9 ). However, if the lower uterine segment cannot be clearly visualized, transvaginal sonography is the most accurate method—the “gold standard”—for assessing the relationship between the inferior placental edge and the internal cervical os (see Fig. 19-9 ). Transvaginal sonography is considered safe regardless of placental location, even in the presence of bleeding. Translabial sonography is less commonly used, but it may be of benefit if transabdominal images are suboptimal and transvaginal sonography is not available or is contraindicated. Abnormalities of placental location include placenta previa and low-lying placenta.

Placenta Previa
Derived from the Latin praevia, for going before , placenta previa indicates that the placenta is the presenting part rather than the fetus. Sonographic identification of placenta previa is essential because affected pregnancies are at risk for vaginal bleeding, which is often initially painless—a herald bleed —but requires urgent evaluation as hemorrhage may ensue. Women with prior cesarean delivery and placenta previa are at risk for placental invasion, and the risk increases with the number of prior cesarean deliveries.
Prevalence
Placenta previa complicates approximately 3/1000 singleton births in population-based series . Risk factors include older maternal age, multiparity, multifetal gestation, cigarette smoking, and especially prior cesarean delivery. Previa is present at delivery in fewer than 1/1500 women younger than age 20 but in more than 1% of those older than age 35. It is largely a complication of multiparous women, as only 20% of cases occur in first pregnancies. It is also more common in twins and is found in approximately 4/1000 twin births. Placenta previa is particularly prevalent among dichorionic pregnancies and attributed to two implantation events covering a larger area.
Terminology
As sonography has replaced physical examination of the cervix in cases of suspected previa, terminology has changed. Largely based on findings from the clinical “double set-up” examination in preparation for delivery of suspected placenta previa, three general categories were formerly used:
- •
Complete placenta previa referred to a placenta that completely covered the cervix, or more specifically, the internal cervical os.
- •
Partial placenta previa indicated that the placenta partially covered the cervical os. Clinically, this could occur and be detected only when cervical dilatation was present, because one had to visualize the placental edge crossing over the dilated cervix.
- •
Marginal placenta previa indicated that the placental edge just reached the margin of the internal cervical os, generally noted via gentle palpation during double set-up examination.

Recently, the classification of placenta previa was revised by a workshop that included the National Institute of Child Health and Human Development (NICHD), Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound. Using the new classification, the diagnosis of placenta previa is made when the placenta covers or just reaches the internal cervical os, eliminating the terms complete, partial, and marginal. If the inferior placental edge encroaches upon the cervix, such that it is within 2 cm of the internal os but does not overlie it, the diagnosis is low-lying placenta . If the inferior placental edge is 2 cm or more from the internal cervical os, the placental location is considered normal.
Placental “Migration”
Most women diagnosed with placenta previa or low-lying placenta in the second trimester will have normal placental location by the end of pregnancy. This apparent “migration” of the placenta away from the lower uterine segment with advancing gestational age has been well described but is not completely understood. Enlargement of the uterus may result in differential growth of the placenta toward the well-vascularized fundus, a phenomenon known as trophotropism. Visualization of the lower uterine segment also may become clearer later in gestation, and placental tissue that previously appeared to have implanted over the cervix, particularly with transabdominal sonography alone, may have been merely adjacent to the region of the cervix and may seem, on follow-up, to have moved several centimeters away.
With transabdominal sonography, approximately 5% of pregnancies are thought to have placenta previa in the second trimester. However, when imaged transvaginally, only 1% to 2% are diagnosed with placenta previa in the second trimester. Overall, about 90% of previas diagnosed prior to 20 weeks’ gestation resolve before delivery. The later in gestation that previa is identified, the higher the likelihood that it will persist until delivery. Specifically, placenta previa identified at about 24 weeks’ gestation persists in approximately half of cases, whereas previa identified at 32 weeks persists in nearly 75%. The degree to which the placenta overlaps the cervical os increases the likelihood that placenta previa will persist, as does a history of one or more prior cesarean deliveries.
Management
Persistent placenta previa will require cesarean delivery. Because of the frequent resolution of previa with advancing gestational age, sonography is indicated if a woman with suspected previa develops bleeding. In the absence of bleeding, follow-up sonography is recommended at approximately 32 weeks’ gestation. If there is any question about placental location, transvaginal sonography should be performed. And, because resolved placenta previa is associated with vasa previa, color and spectral duplex Doppler sonographic scans are recommended to evaluate for possible abnormalities of the umbilical cord insertion. If placenta previa or low-lying placenta are found to persist at the 32-week sonogram, transvaginal sonography is recommended at 36 weeks’ gestation.
Low-Lying Placenta
Placentation is termed low-lying if the inferior placental edge encroaches upon the cervix, such that it does not overlie but is within 2 cm of the internal os ( Fig. 19-11 ). The prevalence of low-lying placenta is approximately 3/1000 pregnancies at approximately 36 weeks’ gestation, thus similar to placenta previa. As with placenta previa, a low-lying placenta identified in the early second trimester often resolves prior to delivery. In one series of 1240 pregnancies with low-lying placenta diagnosed between 16 and 24 weeks’ gestation, 90% had resolved by 32 weeks and 98% had resolved by delivery.

Management
If low-lying placenta is identified in the second trimester, a follow-up sonogram is recommended at approximately 32 weeks’ gestation. If the placenta is low-lying in the third trimester, transvaginal sonography is recommended: (1) to aid in accurate measurement of the distance from the inferior placental edge to the internal cervical os and (2) to exclude vasa previa. If the placenta is low-lying at 32 weeks’ gestation, transvaginal sonography is recommended at 36 weeks.
Unlike placenta previa, low-lying placenta is not a contraindication to vaginal delivery. However, the risks of vaginal bleeding and need for blood transfusion are increased. In studies of women with low-lying placenta who have labored, approximately one third required cesarean delivery specifically for bleeding. In two series, vaginal delivery was more likely if the distance from the placental edge to the internal cervical os (as measured by sonography) was between 1 and 2 cm than if the placenta was within 1 cm of the os. However, whenever the placenta has implanted in the lower uterine segment, even when greater than 2 cm from the internal cervical os, there is an increased risk of bleeding, and excessive bleeding may occur regardless of whether the patient is delivered vaginally or via cesarean.
Placental Cord Insertion Abnormalities
The umbilical cord usually inserts centrally into the placenta ( Fig. 19-12 ). Not uncommonly, however, the cord inserts into the edge of the placenta—a marginal insertion , which is also known as a Battledore placenta (see Fig. 19-12B ). The prevalence of marginal insertion of the umbilical cord is approximately 6% in singleton births and 10% to 15% in twin gestations. Marginal cord insertion, usually defined as cord insertion less than 2 cm from the placental edge, may confer a small increase in the risk for adverse pregnancy outcomes. In a population-based series of more than 600,000 singletons, marginal insertion was associated with placental abruption (odds ratio 1.5), placenta previa (odds ratio 1.8), and small-for-gestational age (SGA) neonate (odds ratio 1.2), but not perinatal deaths. Marginal cord insertion appears to be particularly common in monochorionic twins, and it has been associated with an increased risk for an SGA newborn in monochorionic but not dichorionic twin pregnancies.
Velamentous Cord Insertion
With this abnormality, the umbilical cord does not insert directly into the placenta but rather into the membranes usually a short distance away from the placental edge ( Fig. 19-13 ). Sonographically, the umbilical arteries and vein may be visible traversing the membranes along the wall of the uterus, unprotected by Wharton jelly, before inserting at the margin of the placenta ( Fig. 19-14 ). Color Doppler sonography assists with visualization. Theories regarding the development of velamentous cord insertion include trophotropism, described earlier, or an error in initial blastocyst attachment that orients the fetus a distance away from the decidua basalis rather than directly opposite it.


In population-based series, velamentous insertion occurs in approximately 0.5% to 1.5% of singleton births and 6% of twins. It is more prevalent in monochorionic twins, particularly in the smaller twin of a monochorionic pair, and it is associated with birth weight discordance. In a population-based review of more than 600,000 singleton births, velamentous insertion was associated with an approximately twofold increased risk for placental abruption, SGA neonate, and perinatal death. It has also been reported in 7% of fetal deaths prior to 24 weeks’ gestation. The risk for placenta previa is increased, and this is the most common precursor of vasa previa, discussed next. Care should be taken at delivery to avoid avulsing the umbilical cord when delivering the placenta, as velamentous insertion is associated with a greater than 10-fold increase in need for manual placental extraction.
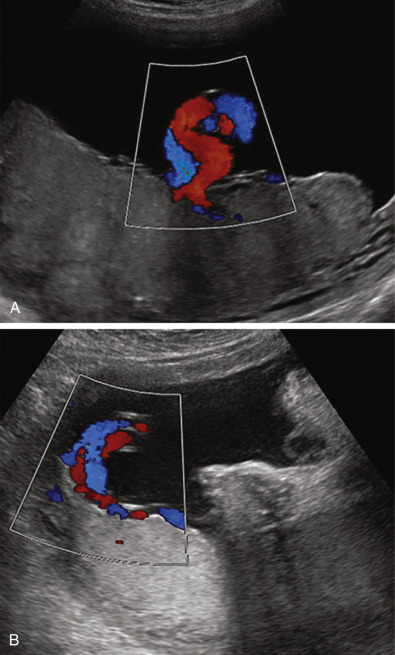
Vasa Previa
In approximately 1/2500 deliveries, fetal vessels course within the membranes that overlie the cervix, a potentially devastating complication termed vasa previa ( Fig. 19-15 ). Vasa previa can develop in two settings: either with a velamentous cord insertion—connecting the umbilical cord to the placenta, or with a succenturiate or bilobed placenta—connecting the two placental lobes. In one review, each of these causes accounted for approximately 50% of prenatally diagnosed vasa previa cases. Multiple gestations are a recognized risk factor, and twins comprise 12% to 25% of those with vasa previa in published series. Pregnancies resulting from assisted reproductive technologies appear to be at particular risk.

There are no standardized criteria for how close the fetal vessels must be to the internal cervical os to constitute vasa previa, and a threshold of “within 2 cm of the os” has been proposed. In one series, all emergent deliveries with vasa previa had a fetal vessel within 2 cm of the internal cervical os.
Vasa previa is potentially catastrophic if unrecognized, because the fetal vessels are prone to compression during labor and because the fetus can quickly exsanguinate when the membranes rupture. With prenatal detection, reported survival rate exceeds 95%. In the absence of prenatal diagnosis, however, the perinatal mortality rate is as high as 50%.
If the diagnosis is suggested sonographically in the second trimester, up to 25% of cases may resolve prior to delivery. If vasa previa persists, scheduled cesarean delivery is recommended prior to the onset of labor. Some have advocated that this occur prior to 36 weeks’ gestation.
Vasa previa should be considered whenever one encounters a low-lying placenta or a resolved placenta previa, as well as in the setting of velamentous cord insertion, succenturiate lobe, or bilobate (bilobed) placenta. Careful sonographic assessment should include color and spectral duplex Doppler evaluation to identify fetal vessels overlying the cervix, which confirms the diagnosis ( Fig. 19-16 ). Routine evaluation of the placental cord insertion site may help to avoid missing this rare but devastating entity. However, it is recognized that even with transvaginal imaging and color Doppler sonography, not all cases of vasa previa will be detected antepartum.

Morbidly Adherent Placenta—Placenta Accreta
Disorders of abnormal placental attachment or invasion include three entities, based on the degree to which chorionic villi invade myometrial tissue:
- •
Placenta accreta : implantation of placental villi directly onto myometrial tissue rather than the decidua basalis
- •
Placental increta : invasion of placental villi into the myometrium
- •
Placenta percreta : placental growth through the myometrium, extending to or through the uterine serosa
At delivery, the placenta fails to separate normally, leading to significantly increased maternal morbidity. Severe hemorrhage may necessitate cesarean hysterectomy and often massive transfusion of blood products with need for intensive care ( Fig. 19-17 ). Thus, to minimize both maternal and neonatal morbidity and mortality rates, prenatal detection is important to permit timely coordination of multidisciplinary care and to arrange for maternal transfer to a center with adequate resources.

Epidemiology and Risk Factors
The prevalence of placental invasion has increased in recent years in parallel with the rise in cesarean delivery rates. Series from the past decade suggest that the prevalence has increased to 1/500 to 1/900 pregnancies.
An overwhelming majority of cases occur in the presence of two factors: placenta previa and prior cesarean delivery. The risk increases sharply with each subsequent cesarean delivery. In the setting of placenta previa, the risk for placental invasion is approximately 3% at the first cesarean delivery, 11% by the second, 40% by the third, and 60% to 70% for subsequent cesarean deliveries. However, in the absence of previa, the risk for placenta accreta is only 0.03% at the initial cesarean and remains less than 1% up to the fifth cesarean. Regardless of whether the placenta previa is anterior or posterior, placental invasion also may develop if the placenta overlies uterine scarring, resulting from prior uterine leiomyoma resection, endometrial ablation, or Asherman syndrome.
Sonographic Findings: Second and Third Trimesters
Sonographic criteria for detection of morbidly adherent placenta or placental invasion include the following:
- •
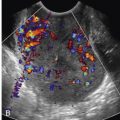
Multiple placental lacunae . These irregularly shaped hypoechoic vascular spaces may give the placenta a “Swiss cheese” appearance. They contain turbulent flow shown with color Doppler imaging ( Fig. 19-18 ).

FIG 19-18
Placental lacunae. A and B, Transvaginal images in the sagittal plane depict placenta previa with multiple placental lacunae. The images are from two different pregnancies, each at 28 weeks of gestation. C and D, Color Doppler ultrasound imaging (from the patients shown in A and B, respectively) demonstrates turbulent flow within these irregularly shaped vascular spaces.
- •
Thinning of the retroplacental myometrium, with the smallest myometrial thickness measuring less than 1 mm in the sagittal plane ( Fig. 19-19 ).

FIG 19-19
Thinning of retroplacental myometrium. This transvaginal image at 32 weeks of gestation shows the placenta (previa) filling the lower uterine segment, and there is marked attenuation of the retroplacental myometrium ( arrows ). The smallest myometrial thickness is below 1 mm.
- •
Irregularity or disruption of the echogenic interface between the maternal bladder and uterine serosa— the bladder-serosal interface —on gray-scale imaging.
- •
Increased vascularity of the bladder-serosal interface, with bridging vessels that course from the placenta to the region of the bladder-serosal interface (and sometimes farther into the bladder) shown with color Doppler imaging ( Fig. 19-20 ).

FIG 19-20
Bridging vessels. This transvaginal sonographic image at 32 weeks’ gestation was obtained in the transverse plane and depicts prominent bridging vessels, shown with color Doppler, that course from the placenta to the bladder-serosal interface. They correspond to the irregularities along the bladder-serosal interface visible with gray-scale imaging.
- •
Loss of the normal linear hypoechoic area between the placenta and the wall of the uterus, called the retroplacental clear space (see Fig. 19-19 ).
Several observations and investigations regarding the sonographic findings of accreta have been reported. Loss of the retroplacental clear space is a common cause of false positive reports and is therefore best used in conjunction with other findings. As single factors, lacunae and interruption of the bladder-serosal interface, particularly when color Doppler sonography confirms hypervascularity, appear to have higher positive-predictive values for invasion than other characteristics. Placental lacunae can vary in degree. Finberg and Williams (1992) developed a grading scale for lacunae: grade 1+ for one to three lacunae, usually small; grade 2+ for four to six lacunae, typically larger; and grade 3+ for diffuse lacunae throughout the placenta. More extensive lacunae throughout the placenta have been associated not just with placental invasion but with placenta percreta. Another finding that may be associated with placenta percreta is hypervascularity of the entire bladder-serosal interface shown with three-dimensional (3D) power Doppler sonography.
In a recent meta-analysis of 23 series that included 3700 pregnancies at risk for placenta accreta, the pooled sensitivity of sonography to identify invasion was 91% and the specificity was 97%. However, the reported sensitivity for the individual studies ranged from 60% to 100%, and the specificity ranged from 50% to 100%. Thus, although the overall utility of sonography for suspected placental invasion is good, there is considerable variability in published reports. More recently, investigators have tried to combine the various sonographic findings with clinical parameters, such as number of prior cesarean deliveries, to more accurately predict placental invasion.
Magnetic Resonance Imaging for Placenta Accreta
Magnetic resonance imaging is not recommended for routine evaluation of suspected placental invasion, as its sensitivity and specificity are similar to sonography, and it has not been shown to affect mode of delivery or need for cesarean hysterectomy. It may be useful, however, when ultrasound findings are inconclusive or additional information is needed ( Fig. 19-21 ). For example, in a patient with prior uterine surgery, instrumentation, or injury involving the posterior myometrium, sonographic visualization may be limited by fetal position or other factors. In such cases in which concern for possible placental invasion is raised, targeted magnetic resonance imaging might be of benefit.

First-Trimester Placenta Accreta and Cesarean Scar Pregnancy
The rising prevalence of placental invasion, coupled with increasing use of first-trimester sonography, has led to recognition of placenta accreta in the first trimester. As previously stated, most pregnancies with placental invasion have placenta previa overlying a prior hysterotomy scar. Similarly, it is possible for implantation to occur in the region of the scar tissue at the anterior lower uterine segment—termed cesarean scar pregnancy —which may result in early uterine rupture. Multiple investigators have found that cesarean scar pregnancy and placenta accreta share the same pathogenesis and that cesarean scar pregnancy, rather than being a separate entity, may be a precursor to second- or third-trimester placenta accreta.
First-trimester sonographic findings of cesarean scar pregnancy include the following:
- •
Gestational sac implantation that is low and anterior in the uterus, in the region of the hysterotomy scar. Before 8 weeks, the sac may fill the “niche” of the scar in a triangular configuration, and by 8 weeks, the gestational sac shape may appear more rounded.
- •
Attenuation of the myometrial layer between the gestational sac and bladder, such that the measured distance from the anterior trophoblastic border to the uterine serosa is 1 to 3 mm ( Fig. 19-22 ). This measurement is comparable to the smallest myometrial thickness reported on sonographic imaging done later in pregnancy.

FIG 19-22
Cesarean scar pregnancy with attenuation of retroplacental myometrium. A, In this transvaginal image of a 6-week gestation, the gestational sac is implanted low and anteriorly in the uterus (in the region of the hysterotomy scar) and appears to bulge outwardly; there is no visible myometrial layer ( asterisks ) between the gestational sac and maternal bladder. B, This transvaginal sonogram at 11 weeks shows thinning of the myometrial layer ( asterisks ) between the gestational sac and the bladder.
- •
Visible irregularities along the placental-myometrial interface.
- •
Echolucent areas within the placenta, a precursor to the lacunae visible later in gestation ( Fig. 19-23 ).

FIG 19-23
Cesarean scar pregnancy with echolucent placental spaces. A, This transvaginal image in the sagittal plane at 10 weeks’ gestation depicts numerous hypoechoic areas within the placenta. Not only has the gestational sac implanted inferior and anterior within the lower uterine segment, but also the sac appears to bulge or “balloon” outward from the uterine serosa. B, Color Doppler sonographic imaging demonstrates prominent turbulent flow within the echolucent areas. This cesarean scar pregnancy was treated with methotrexate and evacuation of the uterus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree