Fig. 18.1
Cyclic ovarian changes: time course for recruitment, selection, and ovulation of the dominant ovarian follicle, with onset at atresia among other follicles of the cohort (Adapted from Hodgen [40])
In the normal ovulatory cycle, the dominant follicle steadily increases in size, while the accompanying smaller follicles are not observed to show a similar increase. Thus, while one or more follicles will grow to full maturity and ovulate, others are destined to atresia and degeneration. This follicular atresia appears to involve genetically programmed cell death within the oocyte – a nuclear cell death referred to as apoptosis.
Ovarian secretion of estradiol (E2) and estrone, from the granulosa cells, promotes follicular maturation by increasing follicular sensitivity to gonadotropin stimulation. This is accepted to be a gonadotropin receptor-mediated process.
The temporal relationship between hormonal profile and follicular development with respect to ovulation is summarized in Fig. 18.2.
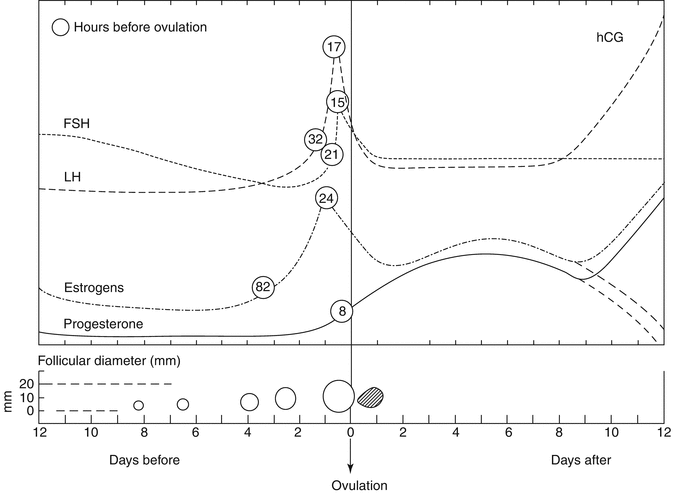

Fig. 18.2
Temporal relationships between hormonal profile and follicular development with respect to ovulation. Significant hormone levels and their preovulatory peaks are given in hours prior to ovulation (circled numbers) (Courtesy of Dr. Josef Blankstein)
The dominant follicle is selected due to its responsiveness to elevated circulatory FSH levels. It is not uncommon to observe two, or more, follicles developing to approximately 10 mm, with one achieving dominance and growing, while the others regress. LH reinitiates meiosis of the oocyte, and typically, ovulation occurs within 36 h of its “surge.”
Small follicles can be visualized easily as echo-free, smooth-walled, structures and usually lie towards the periphery of the more echogenic ovarian tissue. As the follicle matures, more fluid is released and accumulates into its center. The granulosa cell mass, lining the inner of the follicle, increases. Microscopically the oocyte itself, which is less than one-tenth of 1 mm, is surrounded by a cluster of granulosa cells. This complex surrounding the oocyte is termed the cumulus oophorus. It measures approximately 1 mm and can occasionally be depicted by transvaginal scan (TVS) adjacent to the wall of a mature follicle. Immediately prior to ovulation the cumulus separates from the wall and floats freely within the follicle’s center. Today, even with the enhanced resolution afforded by TVS, the attached or floating cumulus is only rarely seen. However, new technological developments, mainly high-resolution probes (40 MHz), have enabled clinical researchers to clearly visualize the antrum, the granulosa (GC), and the theca cells (TC) in a preovulatory follicle (Fig. 18.3).
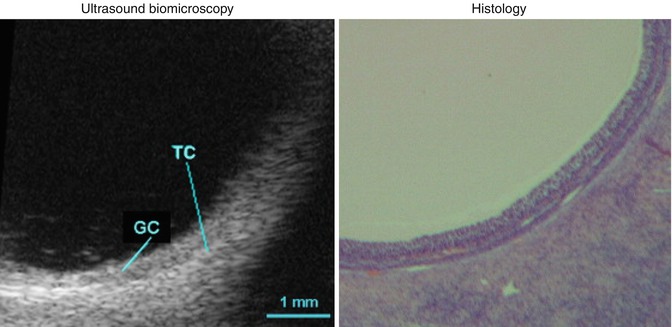

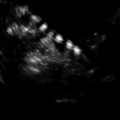
Fig. 18.3
Antrum, granulosa (GC), and the theca cells (TC) in a preovulatory follicle (Reprinted from Palleres et al. [41]. With permission from Elsevier)
Monitoring ovarian response to ovulation induction can be achieved by ultrasonography alone. The dimensions of the growing follicles are plotted from around day 8 of stimulation together with a measurement of endometrial thickness. The mean follicular growth rate is 1.4 mm/day in spontaneous menstrual cycle and 1.7 mm during ovarian stimulation cycles [4].
Mature follicles, those containing a mature oocyte, typically measure from 17 to 25 mm in average inner dimension. The optimal follicular size before triggering ovulation in intrauterine insemination cycles with clomiphene citrate or letrozole was found to be in the 23–28 mm range. The optimal size of the leading follicle was not statistically significantly different between cycles using letrozole or clomiphene citrate and was closely related to the endometrial thickness [5]. Intrafollicular echoes may be observed with mature follicles, probably arising from clusters of granulosa cells that shear off the wall near the time of ovulation. After ovulation, the follicular wall becomes irregular as the follicle becomes “deflated.” The fresh corpus luteum usually appears as a hypoechoic structure with an irregular internal wall and may contain some internal free-floating or fixed echoes that correspond to hemorrhage. As the corpus luteum develops 4–8 days after ovulation, it appears as an echogenic structure of approximately 15 mm in size. Its wall is thickened due to the process of luteinization. TVS shows the neovascularity within the wall that is associated with formation of the corpus luteum. In addition to delineation of changes in follicle size and structure, TVS can depict the presence of intraperitoneal fluid. It is normal to have approximately 1–3 mL of intraperitoneal fluid in the cul-de-sac throughout the cycle. When ovulation occurs, there typically is between 4 and 5 mL within the cul-de-sac. The intraperitoneal fluid resulting from ovulation may be located outside of the posterior cul-de-sac, surrounding bowel loops in the lower abdomen, and upper pelvis or in the anterior cul-de-sac superior to the uterine fundus (Fig. 18.4).
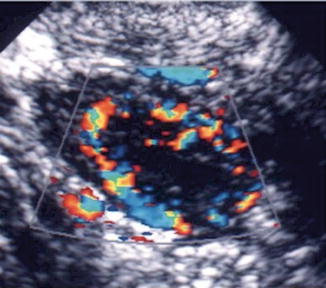

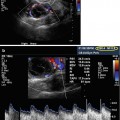
Fig. 18.4
Corpus luteum ultrasound study. (1) Note the irregular cystic mass with crenulated borders and low-level echoes. (2) Doppler findings of a hypervascular corpus luteum with low resistance index
The Role of Doppler in Reproduction
The formation of new blood vessels is taking place in the ovary during folliculogenesis and corpus luteum formation, as well in the endometrium mainly during the follicular phase. It was already recognized as early as in 1926 that neovascularization may be of prime importance in the growth and selection of ovulatory follicles, in addition to the subsequent development and function of the corpus luteum. Studies of ovarian vascular morphology showed that the capillary network of preovulatory follicles was more extensive than that of other follicles, consequently proposing that initiation and maintenance of follicular growth depends on development of the follicular microvasculature.
A study done by Shrestha et al. [6] to determine whether ovarian perifollicular blood flow (PFBF) in the early follicular phase (EFP) is associated with treatment outcome of IVF showed high-grade ovarian PFBF in the EFP during IVF to be associated with a higher clinical pregnancy rate. Coulam et al. [7] correlated peak systolic velocity (PSV) of individual follicles with oocyte recovery, fertilization rate, and embryo quality in women undergoing in vitro fertilization (IVF) and embryo transfer. They assessed the role of quantitative and qualitative indices of follicular vascularity in predicting pregnancy after IVF and embryo transfer. Women who had PSV ≥10 cm/s in at least one follicle on the day of hCG administration more often became pregnant than those with PSV <10 cm/s (P = 0.05). Nargund et al. [8] demonstrated that there was a 70 % chance of producing a grade I or II embryo if the follicular blood velocity was >10 cm/s, compared with 14 % if the PSV was <10 cm/s. This study concluded that there is a physiological relationship between follicular blood velocity, oocyte recovery, and the production of a high-grade preimplantation embryo, which may form the basis of a useful clinical test. Jayaprakasan et al. [9] on the other hand concluded that ovarian vascularity as measured by 3D ultrasound is not decreased in women who demonstrate poor ovarian response to controlled ovarian stimulation as part of assisted reproduction treatment.
Perifollicular vascular perfusion appears to be an important factor in determining the outcome of stimulated cycles, and may have clinical implications in assisted reproduction therapy. As there were low pregnancy rates and oocyte retrieval in the group of women with uniformly low-grade vascularity, the identification of these cycles would be valuable in terms of counseling with regard to the potential outcome in that cycle. Ideally, the identification of these women (who may also be “low recruiters”) earlier in the cycle would be helpful. This could allow the cancellation of treatment after careful counseling, on the basis of perifollicular vascular perfusion, and could be cost-effective, both financially and emotionally. However, further longitudinal data would be needed before this form of prospective management of treatment cycles could be applied clinically. The risk of multiple pregnancies and their implications on the health service is also well recognized. Since there were higher multiple pregnancy rates in stimulated intrauterine insemination (IUI) cycles with uniformly high-grade follicular vascularity, perhaps these cycles in particular should be considered for follicle reduction or even cancellation. This may potentially reduce the number of developmentally competent oocytes that have a higher capability of producing more viable embryos for implantation [10].
In a recent prospective study by Ivanovsky et al. [11], vascular impedance was calculated using the uterine artery and arcuate artery pulsatility resistance and velocity on the day of hCG administration. It was found that optimal uterine receptivity can be accomplished by reduced vascular resistance and increased blood flow. Obviously more studies are needed to confirm their results.
The relationship between endometrial and subendometrial blood flow and pregnancy after intrauterine insemination was examined in a prospective study. The main outcome measured were vascularization index (VI), flow index (FI), and vascularization flow index (VFI) of the endometrium as well as those of the subendometrial region. These measurements were analyzed in relation to IUI outcome, pregnant versus nonpregnant. It was found that the pregnant group had higher endometrium VI, FI, and VFI scores than the nonpregnant group. The subendometrial region VI, FI, and VFI scores did not differ between the groups [12] (Fig. 18.5).
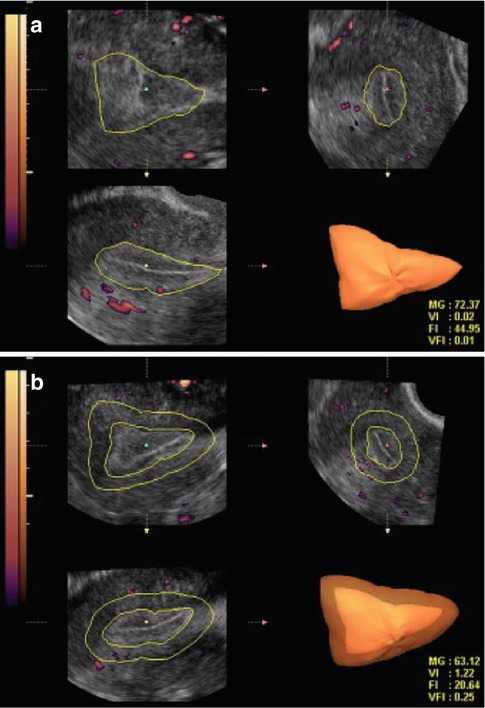

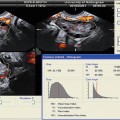
Fig. 18.5
Three-dimensional power Doppler images generated using VOCAL software. (a) Endometrial. (b) Subendometrial blood flow parameters on the day of IUI (see text) (Reprinted from Kim et al. [12]. With permission from Elsevier)
Ovulation Induction and Intrauterine Insemination (IUI)
In conjunction with ovulation induction, IUI is a way to potentially overcome various fertility problems such as oligospermia, i.e., low sperm count, low sperm motility, cervical factor infertility (cervical mucus inactivates sperm motility), sexual dysfunction, and unexplained infertility.
By placing sperm directly into the uterine cavity, the greatest barrier, the mucus in the cervix, is bypassed; therefore, more sperm reaches the egg, creating a better chance of fertilization for the egg. IUI is usually combined with ovulation induction. Optimal timing of insemination is achieved either by the detection of a luteinizing hormone (LH) surge through urinary LH testing (uLH) or by ultrasound monitoring of follicular growth followed by the administration of human chorionic gonadotropin (hCG). In most centers, when the leading follicle reached >18 mm diameter, 10,000 IU hCG was given to trigger ovulation and IUI is timed 36 + or – 2 h later. While IUI is a natural starting point for many treatment schemes, unfortunately this therapy may be complicated by premature luteinization and hyperstimulation.
Premature Luteinization: Premature LH surge will luteinize the follicle which is too small and not ready to ovulate. Cantineau et al. [13] studied the prevalence of premature LH surges in an IUI program. It has been concluded that 24 % of IUI cycles suffer from premature LH surge and this can result in IUI procedure cancellation. Obviously, this represents economic and psychological stress for the patients.
Manzi et al. [14] showed that patients who underwent controlled ovarian stimulation (COS)/IUI treatment and had premature LH surge demonstrated much better pregnancy rates in the subsequent cycle when a GnRH analogue was added, thus avoiding premature LH surge.
GnRH agonist has been, in the past, the standard of care in reducing the incidence of premature LH surge by reversibly blocking pituitary gonadotropin secretion in IUI-stimulated cycles [15]. These drugs are nowadays completely abandoned in IUI cycles because of their stimulatory effect, with consequent higher incidence of multiple pregnancy and OHSS and the long pretreatment period required.
An alternative to GnRH agonists, GnRH antagonists have been proposed to prevent premature LH surge [16]. These drugs do not produce flare-up effect, reducing synchronous follicular pool recruitment. Moreover, the potential advantage of a GnRH antagonist is that pituitary gonadotropin secretion is suppressed immediately after the start of the therapy. Therefore, co-treatment with GnRH antagonists can be restricted to the time in the cycle where there is a risk of premature LH rise.
Multiple Pregnancies
Another concern with controlled ovarian stimulation COS/IUI cycles is the risk of multiple pregnancies. The problem with multiple gestations is that they are associated with major maternal and fetal risks (see Table 18.3).
This past decade has shown increasing medical, societal, and regulatory attention to controlling multiple gestations in all areas of assisted reproduction. Improved outcome-based medical procedures, such as lower gonadotropin dosages, single embryo IVF transfer, and increased utilization of cryopreservation of embryos, have all contributed to the reduction in multiple gestations from ART procedures. Regulatory pressure to lower multiple gestations has come in the form of multiple agencies publishing embryo transfer number guidelines and a national ART tracking database through SART (Society for Assisted Reproductive Technologies). In the USA such regulations remain voluntary, while in many other countries, such guidelines are legislated and strictly enforced.
Low-dose stimulation and careful follicular monitoring may help to reduce the risk of multiple pregnancies. The risk of multiple pregnancies after IUI is dependent on the type of stimulation (clomiphene citrate vs. gonadotropins) and on the size and number of follicles. Dickey et al. [17] reported a positive correlation of multiple pregnancies with the number of follicles 12 and 15 mm or larger. Offering oocyte aspiration of excess follicles in an effort to reduce multiple gestations has been proposed by many researchers, and this method has shown to reduce the risk of multiple pregnancies.
Stoop et al. [18] concluded that aspiration of excess oocytes in stimulated IUI cycles reduced cancellation rates and further reduced multiple pregnancy rates. Additional studies are needed to better define the criteria and methods for oocyte aspiration of preovulatory follicles prior to hCG administration.
Polycystic Ovarian Syndrome (PCOS)
A significant disorder of concern to the reproductive endocrinologist is the polycystic ovary syndrome (PCOS). This is a common cause of anovulation with multiple etiologies. This disorder affects 5–10 % of women. PCOS patients respond well to ovulation induction (see below); however, one has to remember that those patients are prone to develop hyperstimulation and multiple gestations.
For years, PCOS has been one of the most controversial entities in gynecologic endocrinology. Despite a vast amount of clinical and laboratory data that have been accumulated since the initial report of Stein and Leventhal in 1935, our knowledge of the endocrine metabolism underlying the disease is still fragmentary. The PCOS is a disorder of multiple etiologies involving a self-perpetuating imbalance between various interdependent endocrine and peripheral structures. In dealing with patients who exhibit symptoms of the PCOS, we cannot escape the suspicion that we are facing a whole series of interrelated disorders leading to manifestations often classified under this single title (Fig. 18.6).
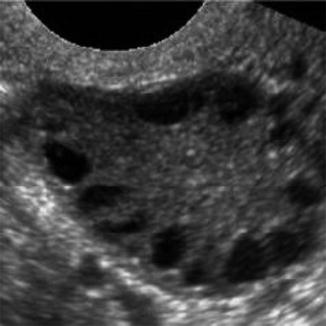

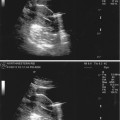
Fig. 18.6
PCOS ultrasound study (note the peripheral small cysts “string of pearls”)
The Classical Picture of PCOS
The PCO syndrome is characterized by a variety of symptoms, all of which are not necessarily present in every patient. These include (1) a broad spectrum of menstrual abnormalities, (2) signs of hyperandrogenism, (3) infertility, and (4) bilateral polycystic ovaries. Menstrual disorders observed include secondary amenorrhea (rarely primary amenorrhea may occur) and oligomenorrhea.
Grossly, the polycystic ovary appears enlarged, sometimes twice the normal size, and is characterized by a shiny, oyster-grey color and small, embedded, bluish cysts (2–6 mm in diameter). Microscopically, the ovarian capsule is thick (approximately 144–595 u wide as opposed to 100 u in normal ovaries) and fibrous and contains numerous primordial follicles. In the substance of the ovary, there are follicles in all stages of development and atresia, and multiple cystic follicles are lined with one to three layers of granulosa cells. Luteinized follicles are present and occasionally corpora lutea have been reported. The walls of the atretic follicles often display hyperplasia of the theca interna cells.
Ultrasound Diagnosis
The criteria for ultrasound diagnosis of PCO have recently been revised in the light of improved ultrasound technology and better understanding of the condition [19]. The diagnosis can be supported when one or more of the following features are demonstrated:
12 or more follicles (2–9 mm diameter) are present in an ovary (either peripheral or diffusely arranged).
Ovarian volume is over 10 cm3 (when no follicles measuring over 10 mm in diameter).
Only a single ovary need be affected to make a diagnosis. If a large follicle is present (over 10 mm), then the volume should be calculated on a repeat scan when the ovary is quiescent to prevent overestimation of ovarian volume.
Remember that imaging findings alone should not diagnose PCOS in an asymptomatic patient. In this situation further supporting evidence in terms of clinical examination and blood tests should be obtained before a firm diagnosis is made. Oftentimes, without clear ultrasonographic or endocrine finding consistent with PCO, the practitioner can still diagnose “suspect PCO” based upon the ovarian response pattern to exogenous gonadotropins, i.e., greater than expected estradiol response and fewer than expected mature follicles – oftentimes, scores of small (less than 10 mm) immature follicles.
Induction of Ovulation
In patients whose infertility can be attributed to an ovulation abnormality, ovulation induction is indicated. Ovulation induction is also used in in vitro fertilization programs (IVF-ET) to increase the number of oocytes aspirated, which in turn increases the number of fertilized conceptus that may be transferred, thereby increasing the chance of pregnancy. Commonly used ovulation induction medications include clomiphene citrate, human menopausal gonadotropin, purified FSH, and recombinant gonadotropins. Although all of these medications result in the development of multiple follicles, they act via different mechanisms.
Transvaginal sonography has a vital role in monitoring the follicular growth rate in women receiving ovulation induction medications.
In an elegant prospective study, Baerwold et al. [4] compared the growth rate of ovarian follicles during natural cycle and ovarian stimulation cycles using standardized techniques.
While the growth rate in natural cycles was 1.42 mm per day, the growth in stimulated cycles was significantly greater, i.e., 1.7 mm per day. Continued research on the effect of greater follicular growth rates and shorter intervals to ovulation is being conducted (Fig. 18.7).
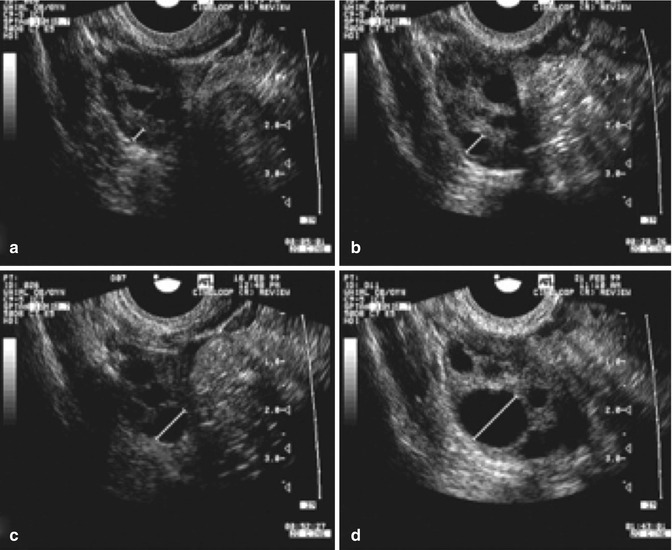
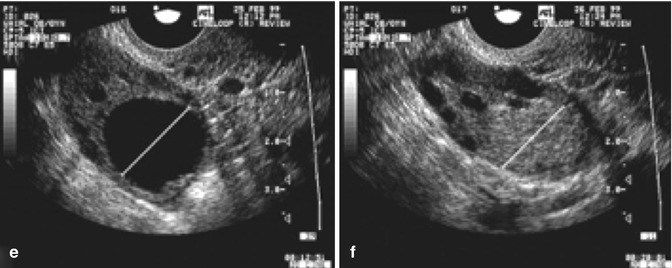


Fig. 18.7
Serial transvaginal ultrasonographic images of the right ovary of a research participant on days 1 (a), 4 (b), 7 (c), 11 (d), 16 (e), and 17 (f) of a spontaneous menstrual cycle. The same ovarian follicle is identified throughout the growth phase in (a–e). The corresponding corpus luteum on the day of ovulation is shown in (e) (Reprinted from Baerwald et al. [4]. With permission from Elsevier)
The baseline scan of the pelvis is mandatory to rule out ovarian or uterine pathology and assess the ovarian reserve: moreover one needs to rule out the presence of ovarian cysts [3].
The objectives of a baseline scan are:
A.

To rule out ovarian or uterine pathology requiring attention prior to beginning infertility treatment (see Table 18.1)
Table 18.1
Common adnexal masses
Cystic masses | Follicular cyst, corpus/luteum cyst, hydrosalpinx, dermoid cyst, endometrioma/hemorrhagic cyst |
Solid masses | Fibroma, dysgerminoma, teratoma, carcinoid subserosal fibroid |
Complex masses | Dermoid cyst, cyst adenoma, granulosa |
A common adnexal finding, endometrosis [20], can be seen in over 30 % of women with clinically defined infertility. Endometriosis is defined as the extrauterine presence of endometrial tissue and is likely due to retrograde menstruation and/or immunologic variations or deficiencies within the peritoneal cavity.
In mild cases small lesions are often located on the ovarian and peritubular surfaces. Cases of minimal endometriosis are not amenable to ultrasonographic diagnosis. However, in more moderate cases, one can visualize an endometrioma, i.e., a cystic structure which is lined with endometrial epithelium which can involve one or both ovaries, uterosacral ligaments, etc.
Endometrioma may appear as an ovarian cyst with an echo-dense appearance of blood within a cyst; the appearance may range from anechoic to solid, depending on the amount and organization of the blood within the cystic structure; commonly one can visualize low-level echoes evenly distributed throughout the cyst (Fig. 18.8).
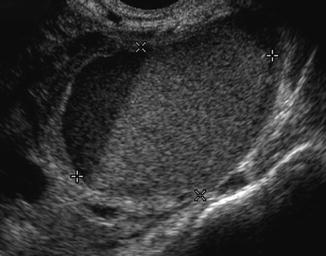

Fig. 18.8
Endometrioma ultrasound study (note the homogenous, low-level echoes, “ground glass” appearance)
It is important for the physicians to familiarize the ultrasonographic picture of the endometrioma in order to avoid aspirating the cyst because of an increased risk of infection, compared with aspiration of a simple cyst.
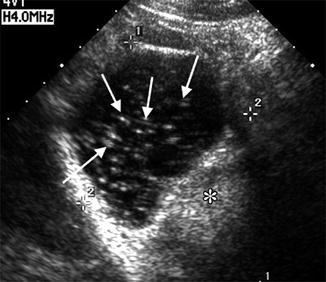
Since ovarian teratomas are the most common ovarian neoplasm especially in reproductive-age women [21], one may encounter them during a baseline scan; the ultrasonographic findings will depend on which elements are present: ectoderm, mesoderm, etc. Very often one can appreciate an echogenic mass with acoustic shadowing. The presence of ectodermal elements gives irregular and variable internal echogenicity (Fig. 18.9).
Get Clinical Tree app for offline access

Fig. 18.9
Teratoma ultrasound study (note the echogenic linear speckles). (asterisk




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree