Fig. 17.1
Ultrasound probes: photo shows a high-frequency, linear array transducer above and a curved array endocavitary transducer below
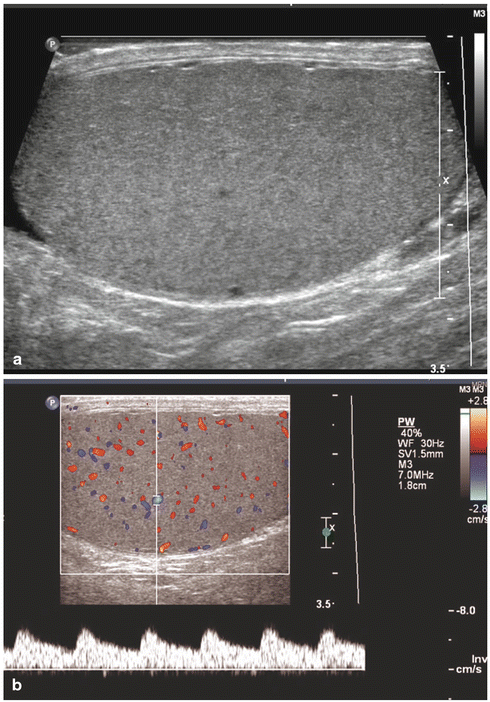
In addition to increasing ultrasound frequency, various forms of Doppler may be utilized to enhance the diagnostic value of the imaging obtained. Power (i.e., color flow) Doppler refers to a form of pulse wave Doppler in which returning echoes are assigned a color (red if moving towards the probe, blue if moving away) so as to differentiate images with velocity (vascular structures) from nonmotile tissue. Duplex Doppler includes the combination of both spectral (flow velocity represented graphically on an X/Y axis) and flow color imaging and is particularly useful to assess the intensity of vascular flow and to assign resistive indices (Fig. 17.2). Additional techniques including elastosonography are under ongoing investigations to determine their clinical utility in routine practice.

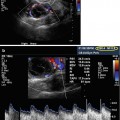
Figs. 17.2
Normal testis: longitudinal sonogram (a) shows the testis to have a homogeneous echogenicity and echotexture. Longitudinal color Doppler sonogram (b) with duplex shows a normal blood flow pattern and normal intratesticular artery velocity tracing
To further discuss the role of ultrasound in the diagnosis and management of male-factor infertility, the current chapter is outlined to review normal and abnormal findings on scrotal and transrectal ultrasonography associated with infertility. When available, standard measurements and anatomic variants are reported. See Table 17.1 for a summary of ultrasound findings associated with male infertility. Brief mention is given to the management of various infertility causes when they relate to pre- and posttreatment ultrasound findings and to the use of ultrasonography with assisted reproductive techniques.
Table 17.1
Ultrasound findings associated with male infertility
Structure | US findings | Associations with infertility |
|---|---|---|
Scrotal ultrasound | ||
Epididymis | Normal caput diameter 7–8 mm | |
Cysts | Hypo-/anechoic, well circumscribed, commonly located at head | Simple cysts (no sperm) and spermatoceles (sperm present) not associated with infertility |
Infections | Enlarged, thickened, decreased echogenicity | MAGI associated with decreased motility, increased sperm DNA fragmentation, abnormal sperm morphology |
Masses | Presence of vascularity, varied echotexture | Most commonly adenomatoid tumors; others include cystadenomas, mesotheliomas, sarcomas |
Obstruction | Epididymal enlargement, prominence of rete testis, hypoechoic appearance | Normal-volume ejaculate with oligo-/azoospermia |
Testicles | ||
Cysts | Hypo-/anechoic, well circumscribed, thin wall | Increased incidence, no known impact on fertility |
Hydroceles | Fluid located between tunica albuginea and vaginalis | Increased incidence, no known impact on fertility |
Infections | Early – decreased echogenicity, increased heterogeneity, enlargement | Associated with subsequent infertility, particularly with postpubertal mumps |
Late – atrophy, increased echogenicity | ||
Masses | Presence of vascularity, varied echotexture | Increased incidence of benign and malignant masses |
Microlithiasis | Increased small focal echogenicity, absence of shadowing | Increased incidence, associated with carcinoma in situ, no known impact on fertility |
Torsion | Early – hyperemia, increased size | Unilateral testicular loss associated with decreased sperm density, increased FSH/LH |
Late – absence of flow, “whirlpool” sign | ||
Trauma | May visualize seminiferous tubules, hematomas | May lead to secondary infertility, antisperm antibodies |
Testicular cord | ||
Masses | Presence of vascularity, varied echotexture | Adenomatoid tumor most common, no known impact on fertility |
Varicocele | Internal spermatic vein ≥3 mm | Decreased sperm count, motility, abnormal morphology, decreased sperm function, varicocele grade inversely associated with sperm density |
Vas deferens | CBAVDa with dilated efferent ducts, prominent epididymal heads, and rete testes | CBAVD found in patients with cystic fibrosis, absence/anomalies of SVs, renal agenesis/anomalies |
Transrectal ultrasound | ||
Prostate | ||
Cysts | May be located peripherally, midline, paramedian, hypo-/anechoic, thin wall | May result in obstruction, rare malignant processes |
Seminal vesicles | ||
EDOa | Dilated ejaculatory duct and SVs, may have calcifications | Low-volume ejaculate, oligo-/azoospermia, decreased fructose and semen pH, requires confirmatory aspiration demonstrating sperm |
Scrotal Ultrasonography
Ultrasound is an optimal imaging modality for the primary evaluation of scrotal pathology. In addition to providing real-time assessments including patient assistance in localization of findings (e.g., pain), advancements in technology permit increasing resolution of underlying structures, assessments of vascular flow, and tissue characteristics (elastosonography). As the scrotum typically does not consist of gas-containing or large calcified structures, a complete visualization of anatomy is available in multiple planes of imaging.
The role for scrotal ultrasonography in the evaluation of the infertile male has been previously established. Scrotal abnormalities have been reported to occur in 38–65 % of infertile men, approximately 60–70 % of which were not found clinically on physical examination alone [15, 16]. In reporting scrotal ultrasound findings in 545 infertile males with a mean age of 36 years, Sakamoto and colleagues identified left varicoceles in 313 (57.4 %), testicular microlithiasis in 30 (5.5 %), epididymal cysts in 21 (3.9 %), right varicoceles in 4 (0.8 %), testicular cysts in 3 (0.6 %), and a testicular tumor, intrascrotal hemangioma, and hydrocele of the spermatic cord in 1 (0.2 %) patient each [16]. When compared to normospermic men, males with infertility have been confirmed to have significantly increased rates of scrotal findings including varicocele (35.5 % vs. 16 %), hydrocele (16.7 % vs. 8.7 %), testicular microlithiasis (9.8 % vs. 2 %), epididymal enlargement (9 % vs. 2.6 %), and epididymal cysts (7.7 % vs. 2 %) [17].
Color flow Doppler adds further value to scrotal ultrasonography as it provides real-time assessments with increased sensitivity to testicular blood flow. This is particularly useful in cases of testicular ischemia, trauma, differentiation of testicular/paratesticular lesions, and infectious processes. Elastosonography, which further assesses tissue firmness, has also been reported to improve characterization of testicular lesions <1 cm [18].
Given the high rate of intrascrotal findings in infertile men, particularly the increased risk of significant pathology such as testicular tumors, scrotal ultrasound is becoming increasingly utilized in the assessment of males presenting with infertility.
Paratesticular Structures
Epididymis
Ultrasound evaluation of the epididymis is performed to assess for the presence of infectious findings, masses or lesions, or evidence of epididymal obstruction. Measurements of the epididymis are obtained at the caput with a normal epididymis measuring 7–8 mm in diameter, with increasing diameter associated with infectious processes [19]. Epididymitis as a clinical diagnosis may be confirmed with ultrasound findings, which include an enlarged or thickened epididymis with decreased echogenicity.
Infectious processes associated with infertility are more broadly categorized as male accessory gland infections (MAGI), which include infections of the epididymis, seminal vesicles, prostate, or bladder. Organisms commonly identified include Chlamydia, Mycoplasma, and E. coli, although organisms such as tuberculosis have also been directly associated with infertility [20]. Although relatively limited data exist and vary by region, the prevalence of MAGI and infertility have been reported to occur in up to 12 % of cases [21]. Several studies have identified abnormal semen parameters in patients with MAGI including decreased motility, increased abnormal forms, and a higher rate of DNA fragmentation [22, 23]. Despite these findings, the etiologic role of MAGI with male-factor infertility remains unclear, as reports have failed to demonstrate consistent findings [24, 25].
Epididymal masses may be further defined as being solid versus cystic. Solid masses are most commonly benign adenomatoid tumors with additional lesions encountered including cystadenoma, mesothelioma, or sarcomas (Fig. 17.3). Cysts of the epididymis are benign lesions commonly located at the head of the epididymis and may represent simple cysts (no sperm in fluid) or spermatoceles (sperm in fluid) (Fig. 17.4). Although epididymal cysts are found more commonly among men with infertility than those without, they have not been shown to result in epididymal obstruction or infertility [17]. In performing surgical resection of spermatoceles and hydroceles, epididymal injury has been reported to occur in 17 and 6 % of cases, respectively [26]. A more recent report by Kauffman and colleagues describing a microsurgical technique of spermatocelectomy demonstrated no changes in sperm count among patients with pre- and postoperative semen analyses, suggesting the absence of iatrogenic epididymal obstruction [27].
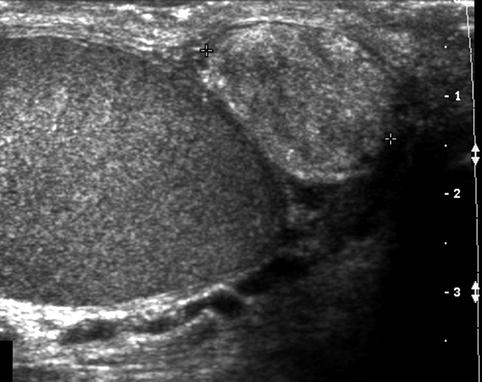
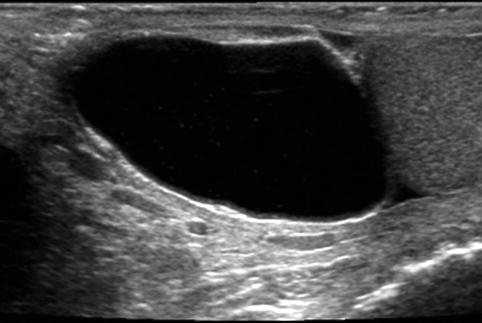

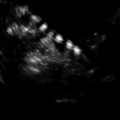
Fig. 17.3
Solid epididymal mass: longitudinal sonogram shows a normal right testis and a solid, heterogeneous mass (between calipers) of the epididymal tail that proved to be an adenomatoid tumor

Fig. 17.4
Cystic epididymal mass: longitudinal sonogram shows a large cystic mass of the epididymal head, along the superior aspect of the testis. Spermatocele is likely a diagnosis, particularly given the few low-level echoes within the mass
In addition to identifying paratesticular masses and infectious processes, improvements in ultrasound resolution have led to its utility in diagnosing epididymal obstruction. Clinical and laboratory findings of epididymal obstruction include normal volume ejaculate with oligo- or azoospermia. Imaging findings may demonstrate epididymal enlargement with prominence of the rete testis and a hypoechoic appearance. Epididymal findings have further been described to help delineate between congenital and acquired causes of obstructive azoospermia. In a report of 211 infertile males undergoing scrotal ultrasonography for obstructive azoospermia, men with a congenital etiology were found to have higher rates of ectasia in the epididymal head with tapering and absence of the epididymal body and tail [28]. Acquired azoospermia, in contrast, exhibited increased rates of epididymal body and tail duct ectasia and an epididymal inflammatory mass.
Varicocele
Varicoceles are reported to occur in approximately 15–25 and 35–60 % of fertile and infertile males, respectively, and remain the most common, reversible cause of male-factor infertility [16, 17, 29]. Clinical varicoceles are more common on the left and are graded on a scale of I–III with grade I varicoceles palpable in the standing position with Valsalva maneuver, grade II palpable in the standing position without Valsalva maneuver, and grade III in the standing position grossly visible. Intratesticular varicoceles identified on ultrasonography are relatively uncommon and are likely of minimal significance for male-factor infertility [30].
Ultrasonography is able to detect varicoceles with a 97 % sensitivity and 94 % specificity [31] (Fig. 17.5). When using the commonly accepted definition of internal spermatic veins measuring ≥3 mm in diameter, ultrasound has been demonstrated to have 53 % sensitivity and 91 % specificity in identifying varicoceles when compared to physical examination [32].
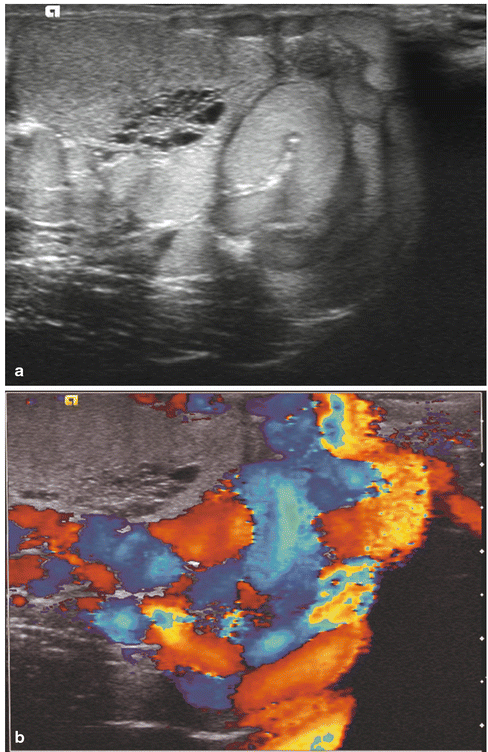

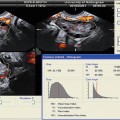
Fig. 17.5
Varicocele: longitudinal sonogram (a) shows multiple serpiginous, dilated scrotal veins. Longitudinal color Doppler sonogram (b) during Valsalva maneuver shows prominent color flow within the vessels
The presence of a varicocele is associated with infertility and impaired semen characteristics including decreased sperm count, motility, and abnormal morphology [33]. In addition, the grade of the varicocele present has been shown to be inversely associated with sperm density [34]. Among infertile patients with a palpable varicocele, only 33.3 % were found to have normozoospermia, highlighting the significant impact on semen characteristics [34]. Similarly, the presence of a varicocele is associated with impaired sperm function with up to 45 % of infertile males with varicoceles demonstrating an abnormal acrosome reaction [35].
Although there is controversy regarding the optimal treatment of males with clinical and subclinical (detected on imaging alone) varicoceles, correction of a palpable varicocele has been consistently shown to improve semen parameters and may prevent progressive decline [36–41]. While the treatment of subclinical varicoceles has not been shown to improve semen characteristics, their presence may be associated with impaired spermatogenesis [42].
A further role for scrotal ultrasonography in the evaluation of patients with clinical varicoceles is the ability to assess and compare testicular volumes. Men presenting with a left clinically palpable varicocele have been shown to have increased rates of ipsilateral testicular atrophy, while subclinical varicoceles have not been associated with discrepant testicular volumes [43]. These findings are significant as adolescents with testicular volume differentials >10 % have been shown to have significantly lower sperm concentrations when compared to those with <10 % differential. This finding was even more pronounced among those with a >20 % differential volume.
Beyond its initial diagnostic role with varicoceles, ultrasonography has further prognostic value in determining paternity success following varicocelectomy. Patients with testicular atrophy were shown to have decreased paternity (11 %) compared to those with normal testicular volumes (30 %) [44]. Similarly, those with clinically apparent varicoceles, bilateral varicoceles, shunt-type varicoceles (both retrograde and antegrade reflux demonstrated on ultrasound), or a permanent degree of varicocele were associated with decreased paternity [44].
An additional study evaluating the impact of preoperative parameters on surgical outcomes demonstrated significant improvements following microsurgical varicocelectomy in sperm concentration, motility, and morphology in patients with testicular vein measurements (taken at the inferior pole of the testis) >2.5 mm compared to veins measuring <2.5 mm [45]. Reflux identified at the inferior pole was similarly associated with improved sperm characteristics compared to those with reflux only identified in the supratesticular venous channels.
Following surgical repair, ultrasound has been reported as a reliable tool in follow-up assessments to document decreased venous diameter at rest and with Valsalva maneuver, although this is of questionable clinical relevance [46].
Vas Deferens
Congenital bilateral absence of the vas deferens (CBAVD) is identified in 1–2 % of infertile males and in approximately 10 % of males with azoospermia [47, 48]. It is found in essentially all patients with cystic fibrosis and is associated with genitourinary abnormalities including absence of the vasal ampulla and seminal vesicles (SV) [49, 50]. Unilateral absence of the vas deferens is associated with both absence (90 % of ipsilateral and 20 % of contralateral) of the SVs as well as SV anomalies including hypoplasia, cysts, and calcifications [49, 51].
Patients found to have an absence of the vas deferens either unilaterally or bilaterally on physical examination can be considered for a confirmatory scrotal ultrasound. Ultrasound findings include absence of the body or tail of the epididymides as well as dilated efferent ducts with associated prominent epididymal heads and rete testis [50, 52, 53]. In the absence of cystic fibrosis, patients with unilateral or bilateral absence of the vas deferens should undergo imaging of the retroperitoneum, as up to 21 or 85 % of patients, respectively, have been reported to have upper tract abnormalities (renal agenesis, renal ectopia, horseshoe kidney) [54, 55].
Testicular Ultrasound
Testicular ultrasonography provides significant information regarding potential etiologies for infertility, identification of prognostic findings, and as a screening modality for associated lesions. Testicular volume assessment may be obtained through various methodologies, with Lambert’s formula (volume [mL] = length × width × AP depth [cm] × 0.71) most commonly utilized [56, 57].
Testicular volume is directly associated with semen parameters including total sperm counts, sperm density, and motility. As seminiferous tubules comprise 70–80 % of testicular volume and are responsible for spermatogenesis, a reduced testicular volume has been correlated with global gonadal dysfunction, as indicated by elevated FSH and LH levels [43, 58–62]. Sakamoto and colleagues noted significant oligospermia in patients with testicular volumes <10 mL (normal 15–20 mL), including length <3.5 cm, depth <1.75 cm, and width <2.5 cm with direct correlations noted with sperm density, total sperm count, motility, and FSH and LH levels [62]. Diminished testicular volume may be secondary to several etiologies including varicoceles, current or previous cryptorchidism, postpubertal mumps, Klinefelter’s syndrome, or hormonal abnormalities, among others.
In addition to estimating testicular volume, Doppler ultrasound may be utilized to identify and assess testicular microcirculation. As spermatogenesis is dependent upon microcirculatory perfusion, diminished testicular blood flow as visualized on ultrasound directly correlates with elevated FSH levels and decreased sperm quality [63–65]. Resistive indices may be obtained to further quantify testicular tissue perfusion and are commonly obtained at the level of the testicular artery and via intratesticular branches near the rete testis. Intratesticular branch resistive indices less than 0.6 have been suggested as a threshold level of normal tissue perfusion, with elevated levels indicative of impaired microcirculation [66, 67].
Testicular ultrasound may assist in differentiating between obstructive and nonobstructive etiologies for infertility. Moon and colleagues demonstrated a reduced median testicular volume in patients with nonobstructive (8.3 mL, range 1.2–16.4) versus obstructive (11.6 mL, range 7.7–25.8) azoospermia [68]. Similarly, patients with azoospermia secondary to obstruction were shown to have dilation of the mediastinum testis, epididymis, and intrascrotal portion of the vas deferens. The sensitivity, specificity, and accuracy for differentiating obstructive versus nonobstructive azoospermia were noted to be 82.1, 100, and 87.5 %, respectively. Further findings which suggest a nonobstructive etiology include reduced or absent testicular vessels, with isolated regions of visualized blood flow potentially indicative of residual spermatogenic production [69].
Cryptorchidism
Cryptorchidism is estimated to occur in approximately 2–5 % of boys born at term and is associated with impaired future fertility [70]. Although there is ongoing debate as to the optimal time for orchiopexy, there is increasing consensus that earlier repair (at 6–12 months of age) results in improved long-term fertility potential [71].
In evaluating future paternity in males previously undergoing orchiopexy for undescended testes, Lee and colleagues observed successful paternity within 12 months in 90 and 65 % of patients with prior unilateral or bilateral cryptorchidism, respectively [72]. This was compared against control subjects who demonstrated a 93 % rate of successful paternity. The author concluded that patients with unilateral cryptorchidism have equal rates of paternity to controls, while patients with repaired bilateral cryptorchidism continue to have impairments in paternity lifelong. Further findings indicated that although patients with unilateral cryptorchidism demonstrated equal rates of paternity, they exhibited elevated levels of FSH, decreased inhibin B, and preserved levels of LH/testosterone compared to controls, suggesting subclinical impairments in spermatogenesis.
To further evaluate the effect of timing of orchiopexy on paternity outcomes among azoospermic patients undergoing IVF, Wiser and colleagues found no difference in rates of sperm retrieval, fertilization, implantation, pregnancy, or live birth rates among men with a history of unilateral (2 patients) or bilateral (40 patients) orchiopexy at ≤10 years of age versus >10 years [73]. Despite the late repairs performed, 60 % of patients were found to have sperm at the time of testicular sperm extraction (TESE).
The role for ultrasonography is likely limited in the initial evaluation of patients presenting with cryptorchidism (Fig. 17.6). Tasian and colleagues performed a meta-analysis to review the diagnostic performance of ultrasonography among patients with non-palpable cryptorchidism with results demonstrating a sensitivity of 45 % and specificity of 78 % in localizing non-palpable testes [74]. These findings increased or decreased in the probability of actually finding an intra-abdominal testicle based on imaging from 55 to 64 % and 49 %, respectively. Given these low rates of precision, the authors indicated that abdominal-scrotal ultrasonography did not reliably assist in the management decision tree for patients with non-palpable testes and was therefore of limited utility. Older patients presenting with non-palpable testes may more reliably undergo MRI in lieu of ultrasound to further assist in localization of intra-abdominal testes.
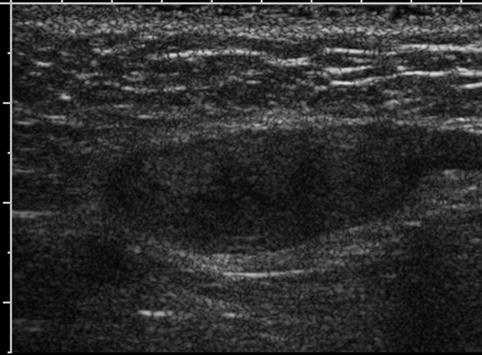

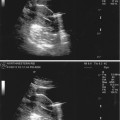
Fig. 17.6
Undescended testis: longitudinal sonogram shows a small, hypoechoic testis in the inguinal canal
Although there is likely limited utility for ultrasound during the initial evaluation of undescended testes, patients with a history of cryptorchidism have a known two- to eightfold increased risk of testicular cancer, with 5–10 % of men with testicular cancer having a prior history of cryptorchidism [70, 75]. This finding has led some authors to advocate for the routine use of scrotal ultrasonography as a screening tool for testicular malignancy among patients presenting with infertility, particularly those with a history of cryptorchidism [76–78].
Cysts, Hydrocele, Infectious Processes
Testicular ultrasonography is an excellent modality for identifying benign testicular structures including cysts, hydroceles, and infectious processes. Intratesticular cysts are identified as hypoechoic/anechoic regions, can represent cystic dilation of the rete testes, and may be a result of postinfectious or posttraumatic epididymal obstruction [79, 80]. Testicular cysts have been reported to occur in 1.2 % of infertile men and are of unclear significance [81].
Scrotal hydroceles represent accumulation of fluid within the tunica vaginalis and are commonly the result of prior trauma, inflammatory, or infectious processes. Although there is a known increased prevalence of hydroceles in infertile males (17 % vs. 9 %), it is unclear if treatment of the hydrocele results in improved semen parameters or fertility [17]. Epididymal injury has been reported to occur in up to 6 % of patients undergoing hydrocelectomy, and this injury may result in impaired fertility, including azoospermia [26, 82]. A long-term follow-up study of children undergoing inguinal hernia repairs demonstrated a 5 % infertility rate, with 15 % of patients previously undergoing hydrocelectomy at the time of herniorrhaphy [83]. To our knowledge, no study has thus far examined the impact of hydrocelectomy on semen parameters in infertile males.
Infectious processes of the testicles visualized on ultrasonography may frequently demonstrate decreased echogenicity, increased heterogeneity, hypervascularity, and testicular enlargement (Fig. 17.7). Similar to MAGI, orchitis may be secondary to infectious (E. coli, Chlamydia, Mycobacterium, mumps, among others) or noninfectious etiologies. Although there remains limited epidemiological data on the impact of orchitis on overall infertility, previous reports have demonstrated oligospermia and azoospermia occurring at follow-up among 15–33 % and 8–27 % of males with unilateral epididymo-orchitis, respectively [84, 85]. Subsequent pathologic analysis of patients with prior epididymo-orchitis has demonstrated scarring of the seminiferous tubules involving both the ipsilateral and contralateral testicles with chronic inflammatory changes noted [84].
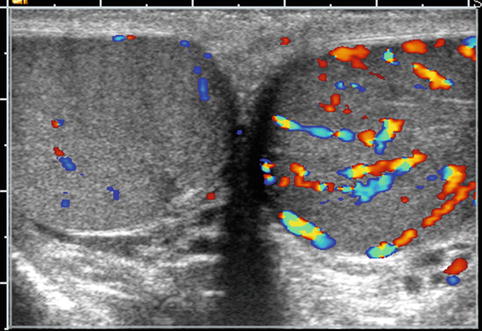

Fig. 17.7
Orchitis: transverse color Doppler sonogram shows both testes with abnormally increased blood flow in the symptomatic left testis
Mumps orchitis is the most common complication of pubertal and postpubertal mumps and is reported to occur in 5–37 % of patients with mumps, with 16–65 % occurring bilaterally [86]. Among patients with a history of mumps orchitis, approximately 50 % will demonstrate some degree of testicular atrophy with one study demonstrating complete atrophy of seminiferous tubules in 38 % of biopsies obtained [24, 87, 88]. These findings may be persistent, even in the setting of appropriate acute phase treatment [88].
Testicular Masses
Males presenting with infertility are at increased risk for both immediate and subsequent development of testicular malignancy (Fig. 17.8). The reported incidence of testicular malignancy in infertile males ranges from 0.2 to 1 % and is estimated to be 20–100-fold more common than in the general population [15, 19, 77, 89–92]. Men with abnormal semen parameters are also at an increased risk of testicular malignancy with an incidence ratio of 1.6 [93]. Additionally, infertile men continue to be at risk for malignancy following sterility with one report of subsequent development of testicular cancer occurring 14 years after initial evaluation [78]. These findings have led some authors to advocate for the routine use of ultrasound during the initial infertility evaluation [76, 77].
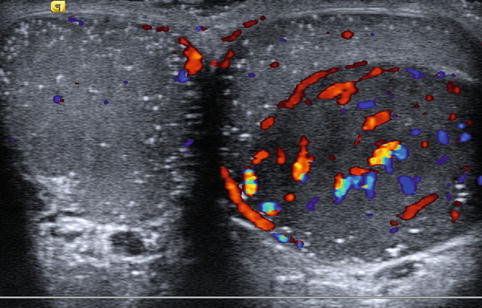

Fig. 17.8
Testicular tumor: transverse color Doppler sonogram shows a large, well-circumscribed hypoechoic mass with prominent blood flow in the left testis and both testes with numerous tiny hyperechoic foci, characteristic of microlithiasis. The mass proved to be a seminoma
The increasing utilization and improved resolution of scrotal ultrasonography have additionally resulted in an increased rate of detection of testicular lesions with series reporting incidental testicular masses in 1–6 % of infertile patients [77, 91, 94, 95]. When the criteria for an incidental testicular lesion are broadened to include hypoechoic/hyperechoic regions, 34 % (49/145) of azoospermic patients are found to have focal abnormalities, with only one of the 49 cases subsequently found to represent malignancy [92].
This increased rate of detection has also led to an altered ratio of benign versus malignant lesions [96]. Carmignani and colleagues reported on a series of patients undergoing scrotal ultrasonography for infertility evaluations as well as multiple causes (varicoceles, testicular pain) with benign pathology found at surgical excision in 75–80 % of incidentally discovered testicular lesions [91, 94]. Other groups have reported higher ratios of malignancies occurring in 50 % (2/4)–71 % (7/9) of incidental lesions, albeit these series were comprised of relatively small numbers [77, 89]. When benign findings are reported on frozen section with later determination of malignancy on final pathology, subsequent orchiectomy specimens were found to have no residual malignancy detected [89].
Given the higher rate of incidental, benign lesions, close observation with repeat physical examinations and ultrasonography has been proposed for non-palpable testicular lesions, particularly those <1 cm [97]. Despite the increasing rate of detection of benign testicular lesions, the decision as to perform radical excision, testicular sparing surgery, or active surveillance remains an area of active debate.
Of interest, testicular ultrasound findings following sperm retrievals including PESA, TESA, and TESE demonstrate focal abnormalities which persist in 77 and 54 % of patients at 5 days and 6 months, respectively [98]. These findings are not to be misinterpreted as concerning for malignancy in this otherwise at-risk population.
Microlithiasis
Microlithiasis is identified on testicular ultrasonography as hyperechoic regions measuring 3 mm or smaller without definitive shadowing present (Fig. 17.9). Among asymptomatic patients undergoing screening scrotal sonography, testicular microlithiasis is reported in 2.4–6 % of individuals with a higher frequency of testicular microlithiasis present in infertile males (10 % vs. 2 % in controls) [99–101]. Despite the known association between testicular microlithiasis and infertility, Yee and colleagues reported no differences noted in semen analyses between infertile males with microlithiasis versus those without microlithiasis [100].
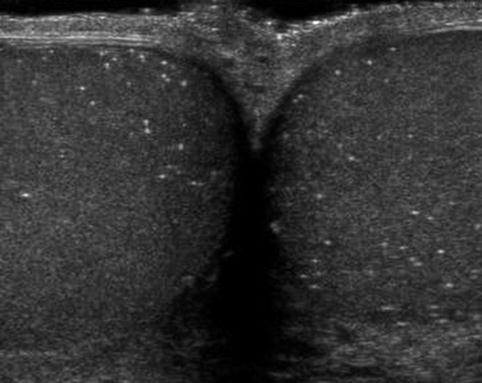

Fig. 17.9
Microlithiasis: transverse sonogram shows multiple diffuse hyperechoic foci with no acoustic shadowing in both testes
Microlithiasis has additionally been associated with a relative risk of testicular malignancy of 21.6, with testicular tumors occurring in approximately 6 % of patients with microlithiasis [102, 103]. Patients with microlithiasis and unilateral testicular germ cell tumors have an increased incidence of carcinoma in situ in the contralateral testicle, with some authors recommending routine biopsy of the contralateral testicle [104, 105]. However, subsequent surveillance of patients with isolated testicular microlithiasis followed over a period of 7 years has not been shown to develop malignancy [104].
Testicular Torsion/Trauma
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree