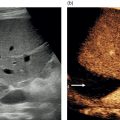
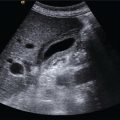
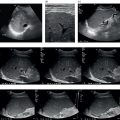
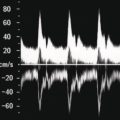
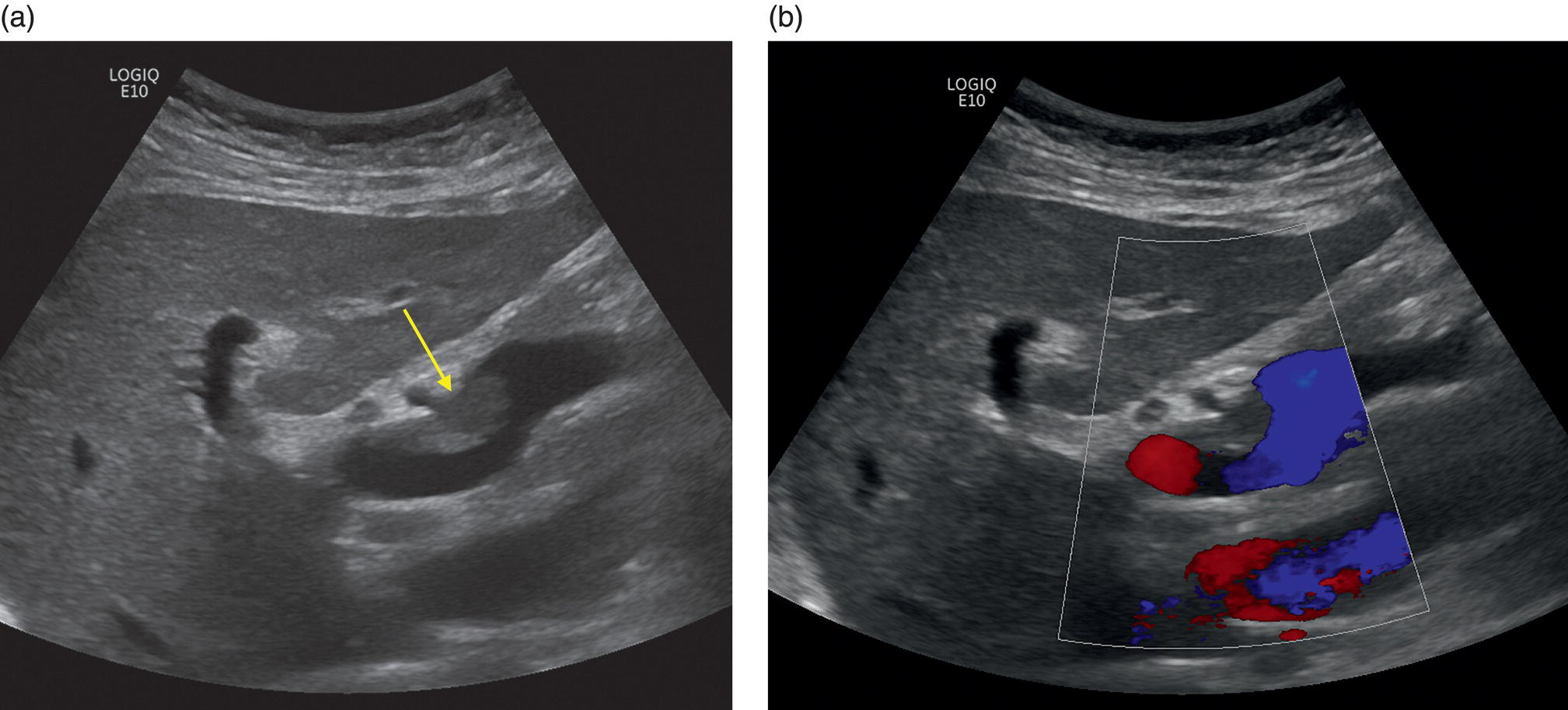
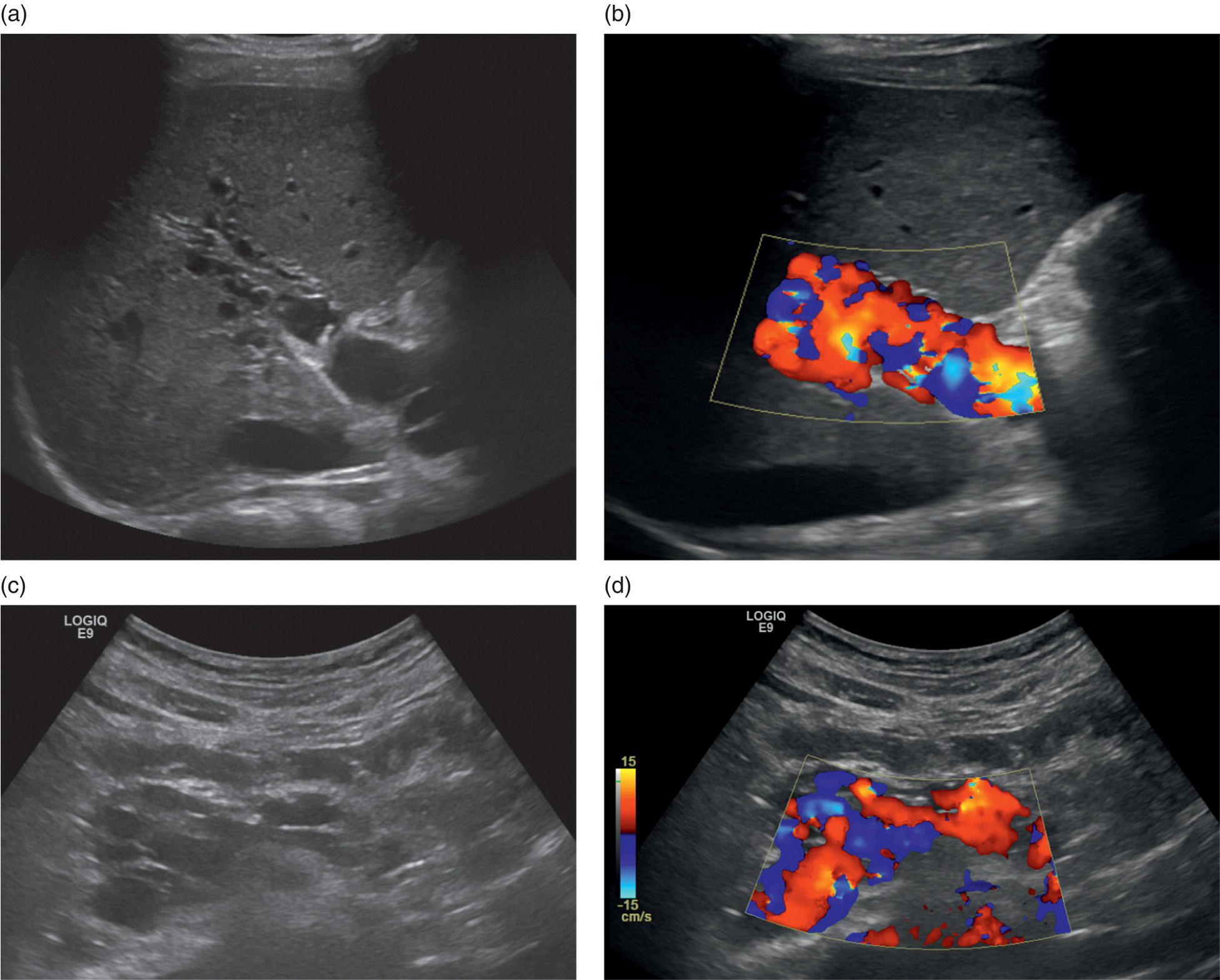
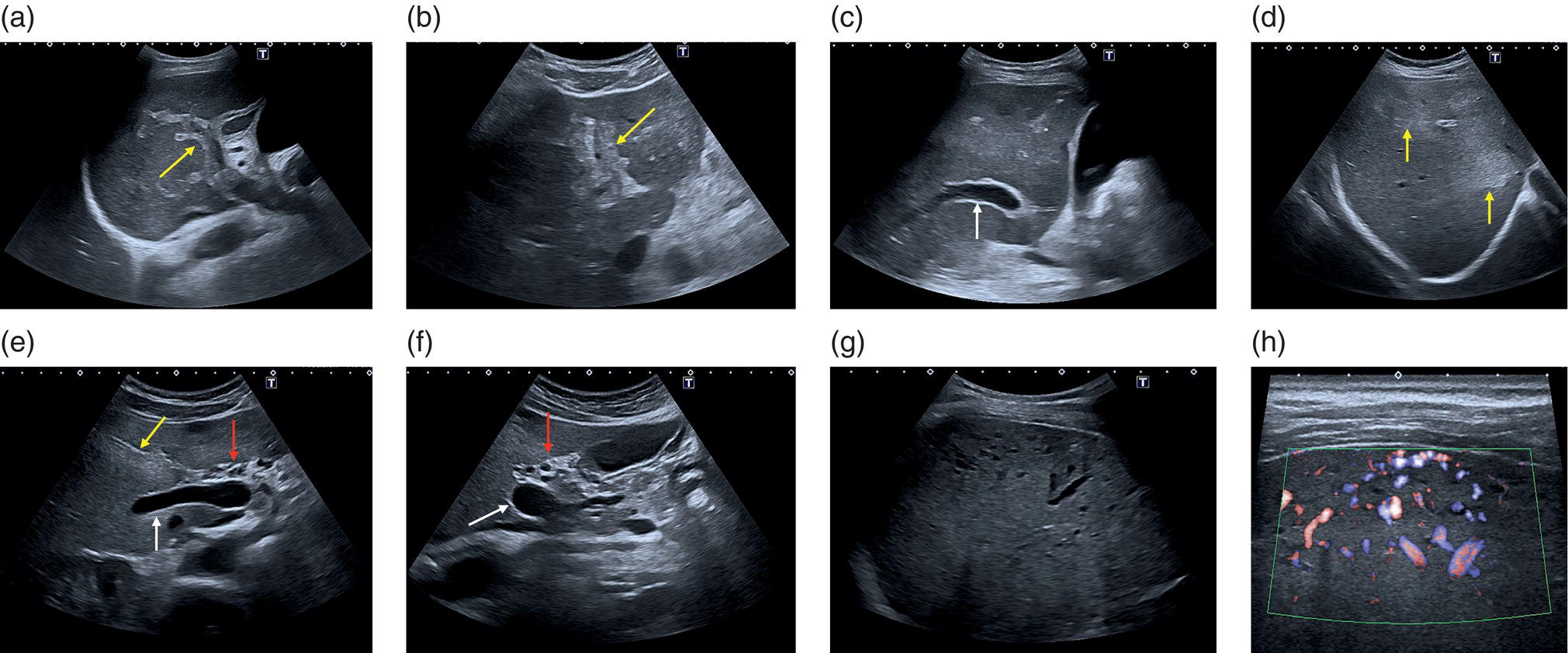
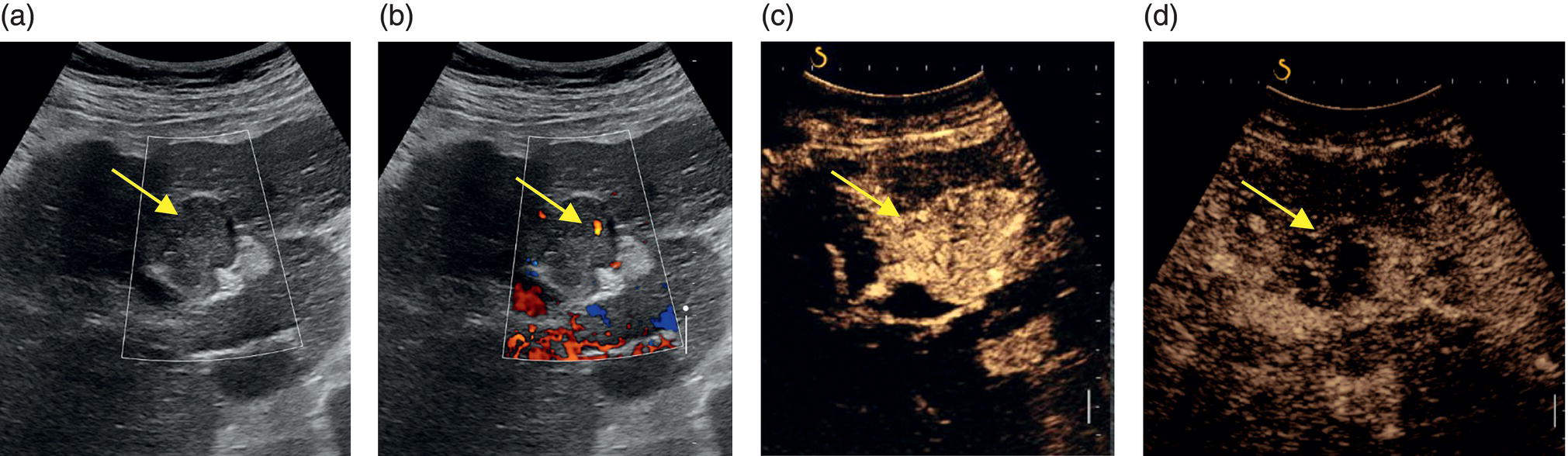
M. Ángeles García‐Criado1 and Annalisa Berzigotti2 1 Radiology Department, Hospital Clínic i Provincial, University of Barcelona, Barcelona, Spain 2 Department of Visceral Surgery and Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland Vascular liver diseases (VLDs) – summarised in Table 11.1 – are a heterogeneous group of diseases affecting one or more of the liver vessels, either at micro‐ or macroscopic level. Many of them are rare or very rare and it is important to note that several VLDs occur in association with, or because of systemic diseases. The latter should therefore be carefully excluded with a VLD diagnosis. The approach and management vary largely according to the type of VLD (see below), ranging from anticoagulation for thrombotic disorders, to interventional radiology techniques (e.g. for large arteriovenous fistulae) and to liver transplantation in selected cases. Among clinically relevant VLDs, thrombotic diseases of the portal vein and of the hepatic veins constitute the largest proportion. In all patients presenting with signs of portal hypertension, thrombotic diseases should be ruled out since they are the second commonest cause of this syndrome after cirrhosis. Ultrasound techniques are very useful in identifying and characterising many VLDs and their associations. Ultrasound is the first modality of choice when there is a clinical suspicion of VLD. This chapter aims to summarise the evidence supporting the use of ultrasound and elastography techniques for the main VLDs. Thrombosis of the portal venous system, or portal vein thrombosis (PVT), is defined by the presence of a clot in the lumen of the vessel at any level (extrahepatic trunk, intrahepatic branches, splenic vein, mesenteric veins), or by the substitution of the vessel with porto‐portal collaterals (cavernous transformation) [1]. Cirrhosis and prothrombotic diseases (either congenital or acquired) are risk factors for the onset of PVT. In cirrhosis, cross‐sectional studies showed prevalences of up to 20% with an incidence of 5–7% per year, while in the general population the risk of PVT is deemed to be <1% in a lifetime [2]. PVT occurring in subjects without cirrhosis is more correctly termed extrahepatic portal vein obstruction (EHPVO) and should prompt a complete work‐up of thrombophilia, including ruling out myeloproliferative neoplasms (JAK2, calreticulin, MPL gene mutations and bone marrow biopsy in selected cases); paroxysmal nocturnal haemoglobinuria (flow cytometry for CD55 and CD59), Behcet’s disease; antiphospholipid syndrome; mutations of Factor II, Factor V, ATIII, Protein C and Protein S, human immunodeficiency virus (HIV) infection, coeliac disease autoimmune and inflammatory systemic diseases [2]. Presentation varies from totally asymptomatic cases identified on imaging done for other causes to abdominal pain, to complications of portal hypertension or intestinal ischaemia. These depend on the severity of occlusion, site of occlusion, duration, extension, and comorbidities. These factors are taken into account in a recent anatomical‐functional classification [3]. On suspicion of PVT, ultrasound should be used as a first‐line technique, since its sensitivity and specificity for thrombosis exceeds 90% [4]. An endoluminal clot or absence of flow or cavernous transformation of at least one of the vessels of the portal system defines PVT. The clot can have an echogenic or hypoechogenic component, and can completely or partially (Figure 11.1) occupy the lumen. The clot is better visualised on greyscale ultrasound but colour and spectral Doppler can help characterise the degree of occlusion (complete/partial with residual flow) [4]. By using the latter techniques, attention should be paid to using the lowest possible scale to avoid false positives owing to slow flow (e.g. in decompensated cirrhosis or in patients on non‐selective beta‐blockers). The cavernous transformation of the portal vein is identified by porto‐portal tortuous collateral vessel substituting (surrounding or within) the thrombosed vessel (Figure 11.2). It can occur at any level of the portal venous system (intra‐ or extrahepatic). Table 11.1 Main vascular liver disorders and role of ultrasound and elastography. Figure 11.1 Partial portal vein thrombosis in a patient without cirrhosis. (a) Note that the clot is better visualised in B‐mode. (b) Colour Doppler further confirms the presence of residual flow in the lumen. In addition, ultrasound not only detects the presence of PVT, but also provides information on factors that may predict its recanalisation with anticoagulation therapy, as well as clinical outcomes [4]. These can be summarised as follows: Figure 11.2 Cavernous transformation of the portal vein. (a) The right intrahepatic portal vein is substituted by hyperechoic (fibrous) tissue, around which small tortuous anechoic channels are visible. (b) Colour Doppler confirms that these are vessels with hepatopetal flow, typical of cavernous transformation. (c, d) A case of cavernous transformation of the extrahepatic portal vein (transversal view in epigastrium). Figure 11.3 These images focus on the vascular changes occuring in the acute (a–d) and chronic phase (e–h) of a portal venous thrombotic event. Acute portal vein thrombosis of the anterior right branch and left branch of the portal vein (a, b yellow arrows). The posterior branch is patent (c, white arrow). Disperfusional changes can be seen within the right liver lobe as subtle ill‐defined hyperechoic areas (d, yellow arrows). The second sequence of images shows the vascular changes occured in the right liver lobe at 3 months from the onset (e–h). The posterior right branch has maintained its patency (e, f white arrow), while the anterior branch is now a fibrotic remnant (e, yellow arrow) and proximal cavernous transformation has taken place (e, f red arrows). Note is made of the appearance of small intrahepatic shunts to bypass low perfusion areas (g, h). Source: Courtesy of Dr Matteo Rosselli. Figure 11.4 Neoplastic complete invasion of the left branch of the portal vein. (a) Note the expansive aspect and the disrupted walls, as well as (b) the presence of signs of arterial flow within the clot. (c) Contrast‐enhanced ultrasound shows enhancement of the thrombus in the arterial phase and in continuity with a large tumour (not visible in B‐mode) and (d) wash‐out in the portal phase, further confirming neoplastic invasion of the portal vein and excluding bland thrombosis. The term ‘porto‐sinusoidal vascular disorder’ (PSVD) has been proposed recently [10] to designate a group of conditions that present histological alterations that involve the portal venules and/or sinusoids without the presence of cirrhosis. It may or may not be associated with portal hypertension. This term groups entities such as idiopathic portal hypertension, idiopathic portal fibrosis, obliterative portal venopathy, hepatoportal sclerosis, incomplete septal cirrhosis, and nodular regenerative hyperplasia (NRH). Specific causes of VLDs are not included in the term. The pathogenesis of PSVD is unknown, but in around 50% of patients it is associated with haematological diseases, immunological disorders, drug exposure, abdominal infections, or congenital defects.
11
Ultrasound in Vascular Liver Diseases
Portal Vein Thrombosis and Extrahepatic Portal Vein Obstruction
Portal vein system
Portal vein thrombosis/extrahepatic portal vein obstruction
Congenital porto‐systemic shunts
Liver sinusoids
Porto‐sinusoidal vascular disorder (idiopathic portal hypertension)
Sinusoidal obstruction syndrome
Diseases affecting the hepatic veins
Budd–Chiari syndrome
Hepatic artery
Hepatic artery diseases (aneurysm, thrombosis, stenosis)
Arteriovenous fistulae
Congenital vascular malformations and hereditary haemorrhagic telangiectasia
Intrahepatic Non‐cirrhotic Portal Hypertension
Porto‐sinusoidal Vascular Disorder/Idiopathic Portal Hypertension
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree