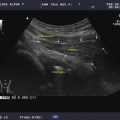
Figure 11.1
The six neck levels are demonstrated. The darker areas are the likely sites for metastatic papillary and medullary carcinoma
Level I
This level has a close relationship to the oral cavity. As such, lymphatic and vascular conditions of the floor of mouth and submandibular gland demonstrate mass lesions in this area. Although it is rare for thyroid carcinoma to metastasize to level I, in the survey of the neck with ultrasound, this region should still be examined. Lymph nodes are commonly enlarged in level I and into the neighboring level II, especially the jugulodigastric node which is ju st cranial and superficial to the carotid bulb (Fig. 11.2). It may be considered in the posterior aspect of zone I or in the cranial portion of level II. Its name derives from the fact that it is located at the place where the posterior belly of the digastric muscle passes over the IJV. While present in most individuals, this commonly hypertrophied lymph node is particularly notable in adolescents and young adults who have experienced recent episodes of tonsillopharyngitis.
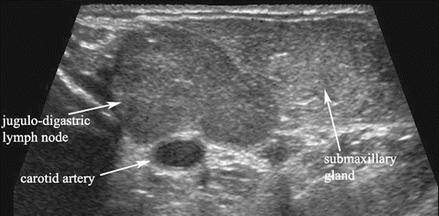

Figure 11.2
This gray scale transverse ultrasound in levels I–IIa demonstrates a hypertrophied jugulodigastric lymph node superficial to the carotid artery
Submandibular Gland
The submandibular gland has a homogeneous ground-glass appearance on ultrasound. It is a discrete structure with a deep lobe that extends deep to the mylohyoid muscle (further away from the skin). It is the most recognizable structure in level I and often demonstrates adjacent benign hypertrophic lymph nodes (Fig. 11.3). The duct structure is not generally identified unless there is an obstructing calculus (stone).
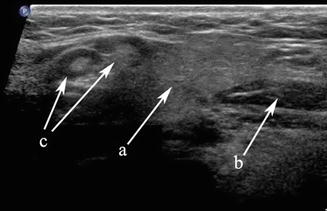

Figure 11.3
The submandibular gland (a), anterior belly of digastric (b), and level I lymph nodes (c) are demonstrated in this transverse gray scale ultrasound
An obstructing calculus of the main (Wharton’s) duct produces dilation and an appearance which could suggest a vascular structure (Fig. 11.4). Doppler is a useful tool to determine that the tubular structure is not vascular. One or more stones can be identified as hyperechoic lines or points with posterior acoustic shadowing as well as the presence of posterior shadowing artifact along the duct structure.
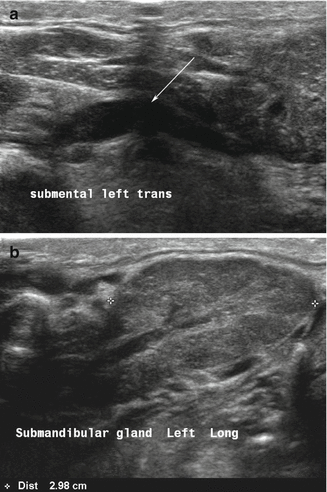

Figure 11.4
(a, b) A distal calculus of the submandibular duct into the floor of mouth produces dilation of the entire ductal architecture as designated by the arrow (a). This ductal dilation or ectasia can be identified even within the gland parenchyma (b)
Common salivary gland diseases can be classified into tumors, inflammatory conditions, and sialosis. A mass within the submandibular gland may be benign or malignant. The sonographic features which favor a benign lesion are a discrete homogeneous mass and a surrounding capsule which clearly separates the lesion from surrounding submandibular parenchyma. The most common benign lesion is a pleomorphic adenoma [2] which demonstrates posterior enhancement; it is one of the few tumors which demonstrate this characteristic so often seen in cysts (Fig. 11.5).
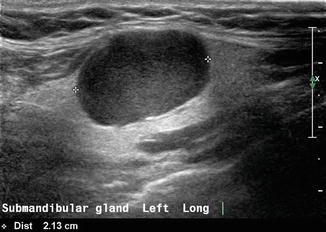

Figure 11.5
A benign mixed tumor (also known as pleomorphic adenoma) of the submandibular gland is demonstrated with gray scale ultrasound. Note posterior acoustic enhancement
The appearance of inflammation of the gland depends on the etiology. Bacterial inflammation can produce decreased parenchymal echogenicity and increased edema, often with dilated ducts. As the inflammation becomes chronic, the gland echogenicity becomes more heterogeneous. Viral infections which are more interstitial are more characterized by heterogeneous and increased echogenicity as well as edematous enlargement. Küttner’s tumor , which has been reclassified as IgG4-RSD, is an excellent example of a chronic sclerosing inflammation of the submandibular gland that often presents bilaterally. Sonographic findings include decreased echogenicity of the parenchyma with heterogeneous, cirrhosis-like hyperechoic bands of fibrosis.
Sialosis is noninflammatory, nonneoplastic often bilateral enlargement of the salivary glands with compressed parenchyma. Later stages of sialosis can mimic chronic inflammation [3]. This condition is frequently seen in patients with bulimia. Fatty “jowls,” or masseter muscle hypertrophy can both resemble parotid gland enlargement, but both of these conditions can easily be distinguished from parotid gland pathology by US. Shrinkage or atrophy and fibrosis of the salivary glands are commonly seen following radiation therapy for head and neck neoplasms. The sonographic appearance is one of small, heterogeneous, hypovascular, and somewhat indistinct glands. Radioactive 131iodine ablation for thyroid cancer is also associated with salivary gland inflammation and injury. In the acute phase, there is often gland enlargement associated with pain; in the chronic phase, there may be ductal obstruction and the appearance of chronic sialadenitis or gland shrinkage and atrophy.
Ranula
A ranula is a cystic enlargement of a portion of the sublingual gland [4]. It may remain situated in the floor of the mouth or can extend posteriorly and deep to the mylohyoid muscle into the neck, and as such is then designated a “plunging ranula (Fig. 11.6).” It is due to an outflow obstruction of one of the sublingual gland ducts. On aspiration, the fluid is relatively clear and has the consistency of saliva. It usually produces a cystic swelling of the floor of mouth and may elevate the mobile tongue.
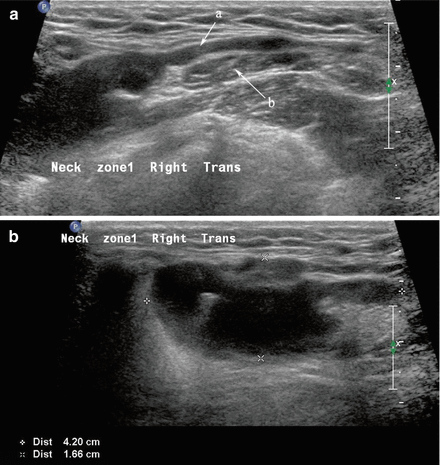

Figure 11.6
(a, b) The hypoechoic homogeneous lesion extending from the sublingual region posteriorly (a) deep to the mylohyoid muscle (b) ends adjacent to the submandibular gland as a dilated cystic structure. The cystic extension adjacent to the submandibular gland in the neck is demonstrated in image (b) where it is designated a “plunging ranula”
Lymphangioma
In contrast to the ranula, a lymphangioma [5] can also produce a cystic mass lesion in level I or floor of mouth, but there are septations and no actual communication with the salivary ducts so it does not contain saliva. Power Doppler is essential to differentiate the lymphangioma from its mate, a hemangioma (Fig. 11.7), and these congenital lesions are collectively known as lymphovascular malformations. A lymphangioma demonstrates its vasculature within the septae rather than within the lesion’s parenchyma. Hemangiomas [6] are also found in the parotid gland, and it is the most common parotid mass lesion in children (Fig. 11.8).
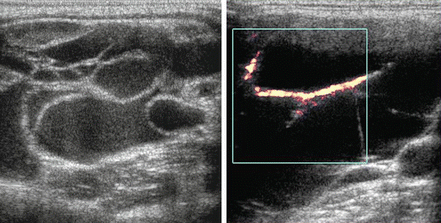
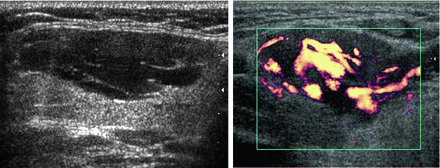

Figure 11.7
Septations within this lesion are indicative of a lymphovascular malformation. Power Doppler demonstrates that the contributory vessels are only within the septae, indicating that the lesion is relatively avascular and consistent with a lymphangioma

Figure 11.8
Septations within this lesion suggest a vascular lesion, but the differential between a lymphangioma and hemangioma cannot be made without Doppler. Power Doppler on the right proves that this lesion is highly vascular and therefore more consistent with a hemangioma
Level II
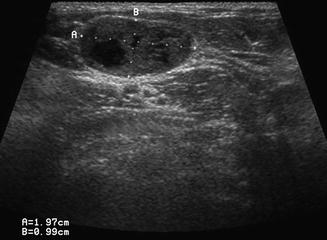
Level II is the most common location for a number of lesions which often cannot be differentiated from one another on physical examination alone. As mentioned previously, the jugulodigastric lymph node is commonly palpated and demonstrated with ultrasound. Usually, sonography demonstrates an appropriate hilum with corresponding hilar vascularity on Doppler both of which suggest likely nodal hypertrophy. A cystic lesion in level II, especially in children and young adults, suggests a possible second branchial cleft cyst [7, 8] in the differential. Fine-needle aspiration cytology is useful to make differential determinations. However, any cystic node in the head and neck must be suspect for metastatic lymphadenopathy (Fig. 11.9). The most common primary origin of a cystic carcinomatous node in level II might be tonsil or tongue base squamous cell carcinoma. Alternatively, thyroid papillary carcinoma can also present as a cystic mass in the neck and even as a solitary mass in level II (Figs. 11.10 and 11.11). The key is to both examine the entire thyroid gland with ultrasound and add thyroglobulin washout to the needle assay of the cystic fluid.
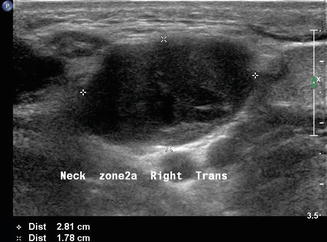
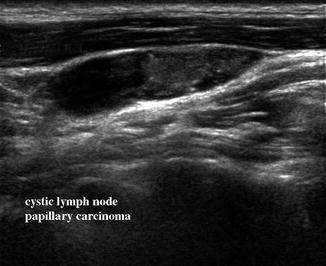
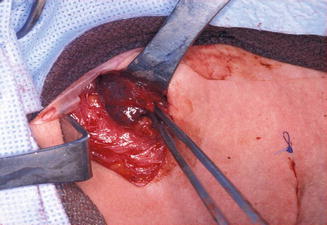

Figure 11.9
The differential diagnosis of a purely cystic lesion within level II includes a second branchial cleft cyst. This hypoechoic mass proved to be cystic on FNA, but cytology demonstrated metastatic squamous cell carcinoma

Figure 11.10
Sagittal view of a lymph node in level II demonstrates cystic changes in its superior region and microcalcifications. This node was proven to be replaced by metastatic papillary carcinoma

Figure 11.11
The surgical field during total thyroidectomy and lateral neck dissection in the same patient as Fig. 11.10. The cystic lymph node with metastatic papillary carcinoma is seen here being delivered from level II
A solid mass in level II may displace the carotid artery into an anterior location. In this circumstance, a lesion of neurogenic origin should be suspected [9, 10]. The cervical plexus or sympathetic chain may be the source of this type of lesion (Fig. 11.12). If a Horner’s syndrome is identified, the lesion is most likely to have originated from the sympathetic autonomic system (Figs. 11.13 and 11.14). If the patient has hoarseness and a vocal cord paralysis, the lesion may have originated from the vagus nerve. In circumstances where a mass resides at the carotid bulb and separates the internal and external carotid arteries, a carotid body tumor or paraganglioma would be realistic considerations [11] (Fig. 11.15). In this circumstance, Doppler demonstrates both the intense vascularity of the mass which is not noted in a schwannoma [12] (Fig. 11.16). The vascular supply to the mass is actually from the ascending pharyngeal artery branch off the external carotid, which can be seen in the lateral Doppler image in this sagittal image (Fig. 11.17). Although none of these lesions are malignant, it is important to understand how to separate them from the more life-threatening clinical conditions.
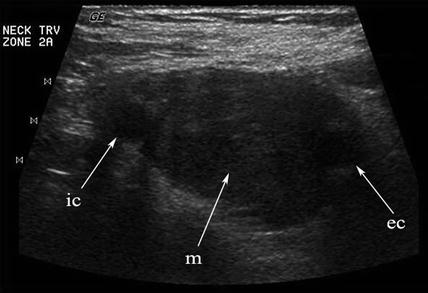
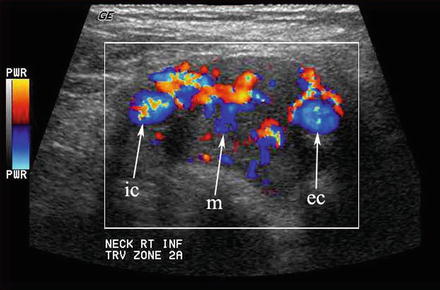
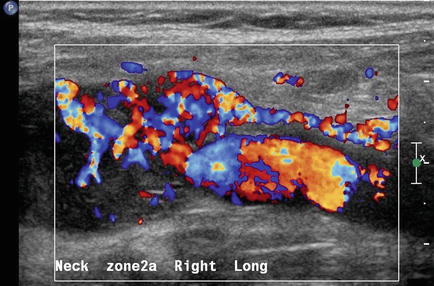

Figure 11.12
A level II cervical rootlet schwannoma is demonstrated. Note the taper of the mass inferiorly which is typical of a neurogenic lesion

Figure 11.13
A patient with a sympathetic chain neurofibroma presents with a Horner’s syndrome

Figure 11.14
This composite image of a cervical plexus schwannoma demonstrates that the actual gross image of the surgical specimen is identically reflected in the preoperative sagittal ultrasound

Figure 11.15
Any hypoechoic mass in level II may be construed as a lymph node or schwannoma. When the mass (m) separates the internal (ic) and external (ec) carotid arteries, a chemodectoma or carotid body tumor (paraganglioma) must be suspected

Figure 11.16
This carotid body tumor located in level II at the carotid bifurcation is clearly defined with color Doppler. The internal (ic) and external (ec) carotid arteries are splayed apart by the vascular mass (m)

Figure 11.17
Sagittal color Doppler image of a carotid body tumor demonstrates its vascular supply from a vessel separate from the common carotid artery. This ascending pharyngeal artery is a branch of the external carotid system
Level III
This region along with level IV is most often associated with conditions relative to the thyroid gland. Although exceedingly rare, lateral aberrant thyroid tissue [13] may be misinterpreted as the more common metastatic lymphadenopathy (Fig. 11.18). These lesions are separate from the main thyroid gland and are homogeneous and more rounded in structure than a lymph node. However, in the context of a mass lesion adjacent to the thyroid gland which is suspect for metastasis, a thyroglobulin washout would be recommended in addition to cytology. If the cytology is benign, no mass lesion of the thyroid gland is identified, and the thyroglobulin is positive; one still must suspect this as a metastatic node from an occult papillary thyroid carcinoma . A formal excision of the mass will be required and in this rare circumstance would confirm this lesion as benign thyroid tissue.
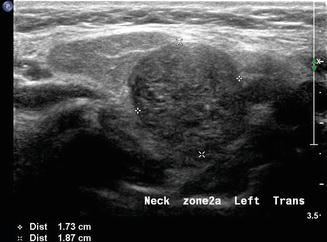

Figure 11.18
A rounded mass in level IIa is demonstrated in transverse gray scale ultrasound. This proved to be one of two lateral thyroid rests which are rare embryonic thyroid remnants completely separate from the thyroid gland. This mass is adjacent to the normal submandibular gland
Patients with recurrent suppurative thyroiditis , especially left sided, may actually have a congenital fourth branchial cleft cyst or fistula which extends from the apex of the piriform sinus ending in the thyroid parenchyma [14, 15] (Fig. 11.19). Episodes may actually begin in children as young as 3 months and present as recurrent retropharyngeal abscess. Neck ultrasound may reveal an inflammatory process overlying the thyroid cartilage in addition to the changes in the adjacent thyroid gland (Fig. 11.20). In some cases, an actual fistulous tract can be identified.
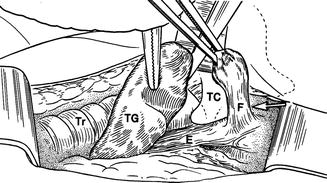


Figure 11.19
This artistic rendering demonstrates a fistula (F) arising from the esophagus (E) and positioned just lateral to the thyroid cartilage (TC). The fistula is invariably left sided and extends to the thyroid gland, frequently into its parenchyma

Figure 11.20
An inflammatory mass lateral to the thyroid cartilage extending into the thyroid gland is due to a fistula extending from the piriform sinus or esophagus (fourth branchial pouch sinus). Note the small circular structure superficial and to the left of the inflammatory mass; this is the actual fistula
The esophagus is an easily recognizable structure deep to the left lobe of the thyroid gland and adjacent to the trachea. Both the muscular and mucosal layers can be identified in the normal state. A Zenker’s diverticulum is a mucosal hernia between the cricopharyngeus and inferior constrictor muscles allowing a pouch to form which traps food and fluid [16–18]. It may be mistaken for a mass lesion. The sonographic features are loss of the usual concentric layers, some enlargement of the apparent esophageal structure, and internal, refractile debris (Figs. 11.21 and 11.22). Either on cine loop or sequential post-swallow static images, the debris often evacuates to some degree. Of course, the ultrasound is simply a means to suspect this lesion, and esophagram is the definitive required imaging study.
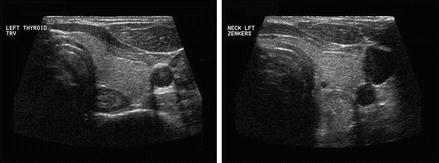
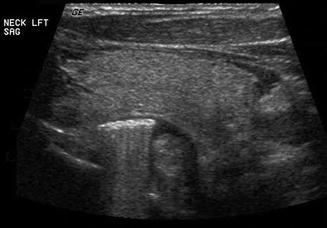

Figure 11.21
The left demonstrates the normal esophagus and the right an expanded esophagus consistent with a Zenker’s diverticulum

Figure 11.22
The sagittal view of this Zenker’s diverticulum reveals food debris which demonstrate refractile sound waves. Note the posterior position of the diverticulum relative to the left lobe of the thyroid gland
Finally, patients with primary hyperparathyroidism can have the source localized with ultrasound. Usually enlarged single hypoechoic lesions suggestive of an adenoma or multiple lesions indicating diffuse hyperplasia in the central neck (level VI) are self-explanatory. When this is noted in the context of hypercalcemia , there is very little confusion about what the images represent. However, a level III nodule in a patient with primary hyperparathyroidism may represent an ectopic parathyroid adenoma (Figs. 11.23 and 11.24). The differential diagnosis always includes lymphadenopathy. The vascularity of a parathyroid adenoma [19] differs from that of a lymph node (Figs. 11.25 and 11.26), and the absence of lymphocytes on aspiration cytology suggests that the lesion is unusual. Parathyroid cytology is notoriously difficult, but an aspirate for PTH will identify the lesion as ectopic parathyroid. Certainly a serum calcium and intact PTH would give an accurate biochemical diagnosis.
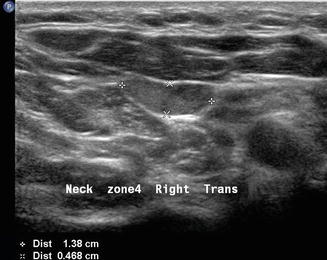
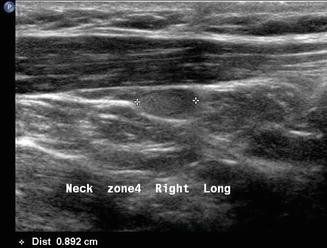

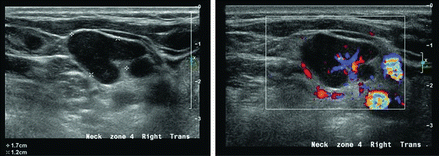

Figure 11.23
A parathyroid adenoma may occasionally be ectopic within the lateral neck, in this instance level III

Figure 11.24
This sagittal view of the ectopic parathyroid adenoma noted in Fig. 11.23 demonstrates its ovoid shape

Figure 11.25
The blood supply to a parathyroid adenoma ends bluntly in the parenchyma without arborization. This small adenoma has the characteristic vascular supply

Figure 11.26
The arborized pattern of vascularity of a hyperplastic level IV lymph node is demonstrated with color Doppler. Note the axial vasculature entering at the nodal hilum which is nicely shown in gray scale on the left
Level IV
Any mass or cystic structure in level IV is suspect for metastatic thyroid carcinoma. The thoracic duct usually enters the venous system on the left side at the posterior junction of the internal jugular and innominate veins. Due to this special anatomical relationship, metastatic malignancy can be identified in this location, especially arising from adenocarcinomas whose origin is from structures below the clavicle. More specifically, the gastrointestinal tract from esophagus to colon may send malignant cells through the thoracic duct to the neck. In addition, other abdominal and pelvic primary tumors such as pancreatic and testicular cancer can produce the same metastatic result. Metastatic squamous cell carcinoma in addition to thyroid cancer should be suspected, especially in adults, when a partially or completely cystic mass is identified in the upper, mid-, or lower jugular chain [20] (Figs. 11.9 and 11.27).