Key points
- •
A multiplicity of acute small infarcts in different vascular territories is a common manifestation of cerebral embolism.
- •
Diffuse white matter blooming foci on T2* MR imaging are considered a pathognomonic imaging pattern of cerebral fat embolism in the setting of bone fractures.
- •
Calcified cerebral embolism carries a high risk of recurrent embolic events in almost one-half of the patients.
- •
Using thin-slice thickness and window settings adjustment may improve visualization of cerebral air emboli on noncontrast computed tomography.
- •
Microbleeds are the most common asymptomatic manifestation of cerebral septic embolism in infective endocarditis.
Introduction
The heart and the carotid arteries are the most common sites of origin of embolic disease to the brain. These emboli consist mainly of red blood cells, platelets, and fibrin. However, there are other less common cerebral emboli with different composition, including air, fat, calcium, and tumor cells. Some of these emboli could have a different site of origin, including the venous system. Although infarcts can be the final result of any type of embolism, here are described the ancillary and sometimes unique imaging features of less common types of cerebral emboli that may allow for a specific diagnosis to be made or at least suspected in many patients.
General Features of Cerebral Emboli
Emboli travel distally through progressively narrower arteries before lodging where the blood vessel becomes too small for further progression, especially at branching points. Chung and colleagues have shown that large emboli strongly favor the middle cerebral artery (MCA), while smaller emboli are carried proportionally to flow volume in the MCA and anterior cerebral artery (ACA) territories.
Emboli lodging in the proximal intracranial arteries result in territorial cortical infarcts, whereas smaller emboli lodging distally in terminal cortical branches result in small wedge-shaped peripheral infarcts centered at the gray-white matter interface. Uncommonly, embolic lacunar infarcts may occur in conditions causing massive showers of emboli. Overall, emboli are more likely to travel toward the leptomeningeal arteries rather than to the small perforators due to the smaller caliber and sharp angles of the perforators as they arise from parent vessels.
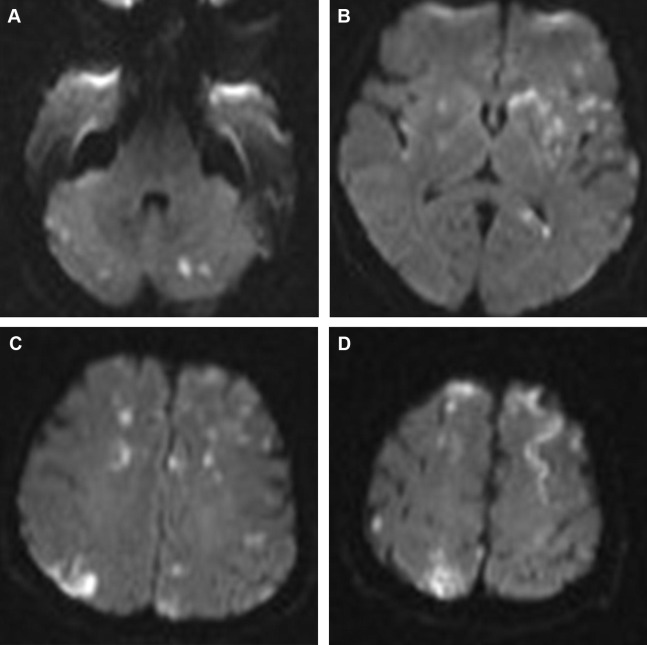
A multiplicity of acute small infarcts in different vascular territories suggests emboli and is best appreciated on the diffusion-weighted imaging (DWI) ( Fig. 1 ). However, multiple acute infarcts can also be seen with watershed infarcts, vasculitis, hypercoagulable states, and in presence of anatomic variants connecting the anterior and posterior circulations (eg, fetal posterior cerebral artery [PCA] and persistent trigeminal artery) or connecting both hemispheres (eg, azygos A2 supplying the ACA territories bilaterally). Of note, multiple acute infarcts exclusively affecting the posterior circulation are, in most cases, related to large-artery atherosclerosis.
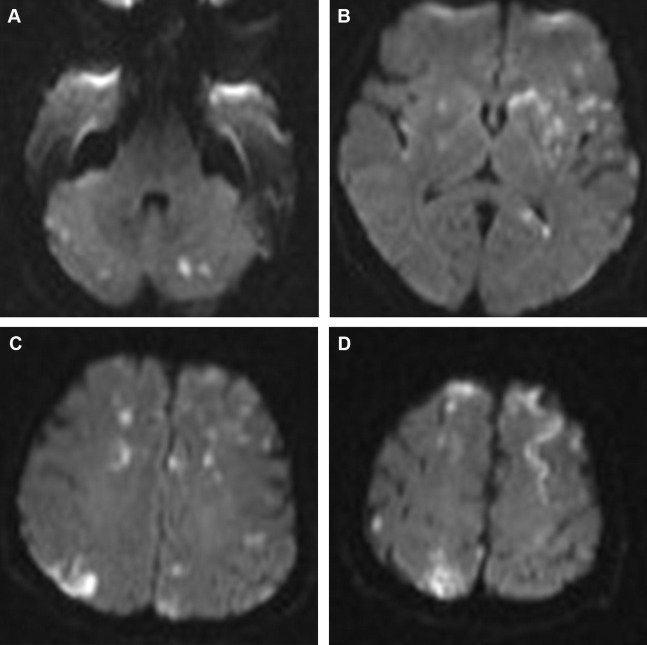
Hemorrhagic transformation is a common feature of embolic infarcts and is best seen on susceptibility blood-sensitive MR imaging sequences as petechial hemorrhage as opposed to the larger parenchymal hematomas that develop more commonly in territorial infarcts such as those involving the MCA.
Cerebral Fat Embolism
Cerebral fat embolism (CFE) is a consequence of fractures, especially those involving the lower extremity long bones and the pelvis, orthopedic procedures, as well as nontraumatic conditions including bone infarcts in sickle cell disease and acute hemorrhagic pancreatitis. It presents as a triad of pulmonary and neurologic dysfunction and petechial rash. The neurologic presentation varies from asymptomatic to subclinical to headache, confusion, and coma. The onset varies from a few hours up to 48 hours after trauma with a mean of 29 hours. Clinically apparent fat embolism occurs in 0.5% to 3.5% of isolated long bone fractures and in 5% to 10% of polytrauma patients. Subclinical fat embolism is thought to occur in all cases of lower extremity and pelvic fractures. The incidence of CFE is 0.9% to 2.2% of patients with a long bone fracture.
CFE is thought to result from the exposure of medullary fat to damaged blood vessels with fat mechanically forced into the venous system. An alternative theory suggests that stored fat is mobilized into the plasma due to increased levels of catecholamines and plasma lipase, resulting in fat droplets throughout the entire circulation. Fat emboli cross the pulmonary circulation and reach the intracranial circulation even without an intracardiac shunt being present.
Intracranially, fat emboli lodge in and mechanically occlude capillaries, producing ischemia and cytotoxic edema. However, the fat globules are deformable and tend to break up into smaller ones, which recycle through the pulmonary circulation. Hence, occlusions tend to be temporary, and changes may be reversible. Cytotoxic edema may also result from the release of toxic metabolites (oleic acid and triglyceride triolein). The release of toxic metabolites also results in opening of the blood-brain barrier (BBB), causing vasogenic edema and enhancement on contrast studies.
Imaging Findings
The “star field” appearance ( Fig. 2 ) is the most common imaging pattern found, and yet it remains nonspecific. It is seen on DWI early in the acute phase as multiple foci of high signal scattered throughout the brain predominantly in the border zones and deep gray nuclei bilaterally. These foci are thought to represent cytotoxic edema in acute cerebral infarcts. As the occlusions are temporary, the findings can be reversible, leading to better outcome compared with other cerebral embolic events. A second pattern seen on DWI, usually in the subacute phase, is the confluent bilateral symmetric periventricular and subcortical white matter cytotoxic edema and diffusion restriction. This pattern portrays a worse prognosis.
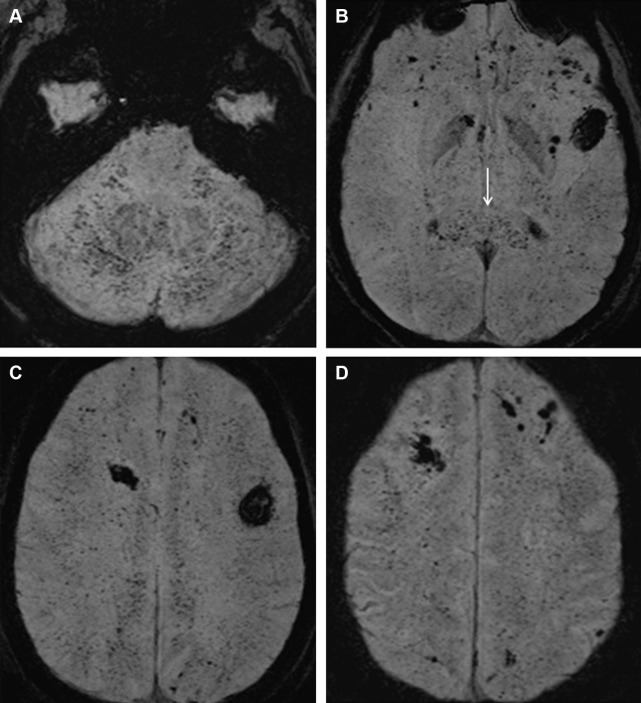
T2* sequences may reveal innumerable foci of blooming low signal in the white matter ( Fig. 3 ). This pattern is best appreciated on susceptibility-weighted images (SWI) and is to be pathognomonic of CFE. The diffuse microhemorrhages can be secondary to microscopic hemorrhagic infarcts and vessel wall rupture due to embolism or leakage of red blood cells because of abnormal BBB. Alternatively, Suh and colleagues suggested decreased blood flow secondary to embolism, leading to local increased deoxyhemoglobin levels as an underlying explanation for the extensive abnormalities seen on SWI.
White-matter T2/FLAIR (fluid attenuation inversion recovery sequence) hyperintense foci with no diffusion restriction can be seen, can reflect vasogenic edema, and may show contrast enhancement. T2 abnormalities appear later than the DWI changes and are nonspecific. However, the number of white matter lesions on T2-weighted images correlates with the patient’s Glasgow coma scale score. Magnetic resonance (MR) spectroscopy shows the presence of lipid peaks within the lesions, a finding related to the nature of the emboli or associated necrosis.
Introduction
The heart and the carotid arteries are the most common sites of origin of embolic disease to the brain. These emboli consist mainly of red blood cells, platelets, and fibrin. However, there are other less common cerebral emboli with different composition, including air, fat, calcium, and tumor cells. Some of these emboli could have a different site of origin, including the venous system. Although infarcts can be the final result of any type of embolism, here are described the ancillary and sometimes unique imaging features of less common types of cerebral emboli that may allow for a specific diagnosis to be made or at least suspected in many patients.
General Features of Cerebral Emboli
Emboli travel distally through progressively narrower arteries before lodging where the blood vessel becomes too small for further progression, especially at branching points. Chung and colleagues have shown that large emboli strongly favor the middle cerebral artery (MCA), while smaller emboli are carried proportionally to flow volume in the MCA and anterior cerebral artery (ACA) territories.
Emboli lodging in the proximal intracranial arteries result in territorial cortical infarcts, whereas smaller emboli lodging distally in terminal cortical branches result in small wedge-shaped peripheral infarcts centered at the gray-white matter interface. Uncommonly, embolic lacunar infarcts may occur in conditions causing massive showers of emboli. Overall, emboli are more likely to travel toward the leptomeningeal arteries rather than to the small perforators due to the smaller caliber and sharp angles of the perforators as they arise from parent vessels.
A multiplicity of acute small infarcts in different vascular territories suggests emboli and is best appreciated on the diffusion-weighted imaging (DWI) ( Fig. 1 ). However, multiple acute infarcts can also be seen with watershed infarcts, vasculitis, hypercoagulable states, and in presence of anatomic variants connecting the anterior and posterior circulations (eg, fetal posterior cerebral artery [PCA] and persistent trigeminal artery) or connecting both hemispheres (eg, azygos A2 supplying the ACA territories bilaterally). Of note, multiple acute infarcts exclusively affecting the posterior circulation are, in most cases, related to large-artery atherosclerosis.
Hemorrhagic transformation is a common feature of embolic infarcts and is best seen on susceptibility blood-sensitive MR imaging sequences as petechial hemorrhage as opposed to the larger parenchymal hematomas that develop more commonly in territorial infarcts such as those involving the MCA.
Cerebral Fat Embolism
Cerebral fat embolism (CFE) is a consequence of fractures, especially those involving the lower extremity long bones and the pelvis, orthopedic procedures, as well as nontraumatic conditions including bone infarcts in sickle cell disease and acute hemorrhagic pancreatitis. It presents as a triad of pulmonary and neurologic dysfunction and petechial rash. The neurologic presentation varies from asymptomatic to subclinical to headache, confusion, and coma. The onset varies from a few hours up to 48 hours after trauma with a mean of 29 hours. Clinically apparent fat embolism occurs in 0.5% to 3.5% of isolated long bone fractures and in 5% to 10% of polytrauma patients. Subclinical fat embolism is thought to occur in all cases of lower extremity and pelvic fractures. The incidence of CFE is 0.9% to 2.2% of patients with a long bone fracture.
CFE is thought to result from the exposure of medullary fat to damaged blood vessels with fat mechanically forced into the venous system. An alternative theory suggests that stored fat is mobilized into the plasma due to increased levels of catecholamines and plasma lipase, resulting in fat droplets throughout the entire circulation. Fat emboli cross the pulmonary circulation and reach the intracranial circulation even without an intracardiac shunt being present.
Intracranially, fat emboli lodge in and mechanically occlude capillaries, producing ischemia and cytotoxic edema. However, the fat globules are deformable and tend to break up into smaller ones, which recycle through the pulmonary circulation. Hence, occlusions tend to be temporary, and changes may be reversible. Cytotoxic edema may also result from the release of toxic metabolites (oleic acid and triglyceride triolein). The release of toxic metabolites also results in opening of the blood-brain barrier (BBB), causing vasogenic edema and enhancement on contrast studies.
Imaging Findings
The “star field” appearance ( Fig. 2 ) is the most common imaging pattern found, and yet it remains nonspecific. It is seen on DWI early in the acute phase as multiple foci of high signal scattered throughout the brain predominantly in the border zones and deep gray nuclei bilaterally. These foci are thought to represent cytotoxic edema in acute cerebral infarcts. As the occlusions are temporary, the findings can be reversible, leading to better outcome compared with other cerebral embolic events. A second pattern seen on DWI, usually in the subacute phase, is the confluent bilateral symmetric periventricular and subcortical white matter cytotoxic edema and diffusion restriction. This pattern portrays a worse prognosis.
T2* sequences may reveal innumerable foci of blooming low signal in the white matter ( Fig. 3 ). This pattern is best appreciated on susceptibility-weighted images (SWI) and is to be pathognomonic of CFE. The diffuse microhemorrhages can be secondary to microscopic hemorrhagic infarcts and vessel wall rupture due to embolism or leakage of red blood cells because of abnormal BBB. Alternatively, Suh and colleagues suggested decreased blood flow secondary to embolism, leading to local increased deoxyhemoglobin levels as an underlying explanation for the extensive abnormalities seen on SWI.
White-matter T2/FLAIR (fluid attenuation inversion recovery sequence) hyperintense foci with no diffusion restriction can be seen, can reflect vasogenic edema, and may show contrast enhancement. T2 abnormalities appear later than the DWI changes and are nonspecific. However, the number of white matter lesions on T2-weighted images correlates with the patient’s Glasgow coma scale score. Magnetic resonance (MR) spectroscopy shows the presence of lipid peaks within the lesions, a finding related to the nature of the emboli or associated necrosis.
Calcified cerebral emboli
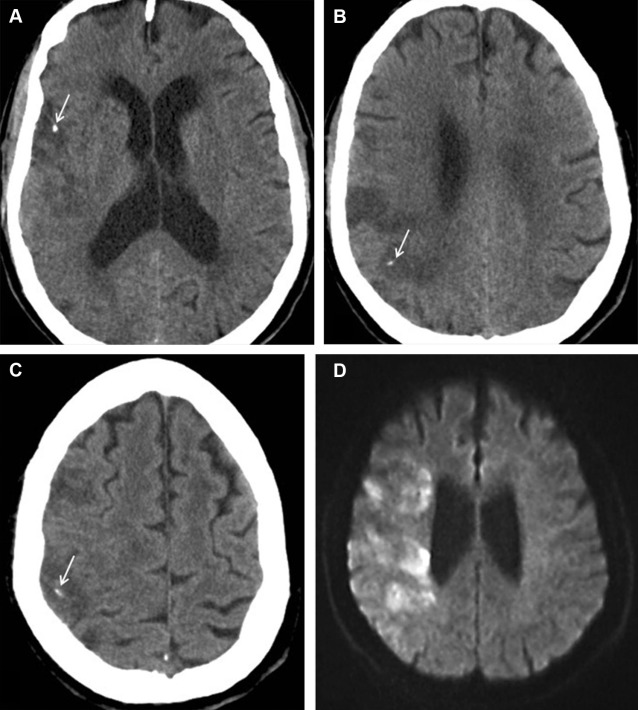
Calcified cerebral embolism is an uncommon cause of stroke that has been recently estimated to occur in 3% of patients assessed for stroke and carries with it a high risk of recurrent embolic events in almost one-half of the patients. Calcified emboli can occur spontaneously or secondary to surgery and catheterization. The sources of these emboli are the heart (especially calcified valves and calcified thrombus), the aortic arch, and neck arteries, including the carotid as well as the brachiocephalic and vertebral arteries. The most common sources are, however, a calcified aortic valve and carotid atherosclerosis.
On computed tomography (CT), calcified emboli are small (2–3 mm) round or ovoid hyperdensities (162 HU) ( Figs. 4 and 5 ). The “salted pretzel” sign describes a near pathognomonic unenhanced CT appearance of multiple calcified peripheral emboli in arteries along the pial surface of the brain, like salt on a pretzel. Differential considerations include arterial wall calcification, which appears more linear than calcified emboli, and other causes of superficial calcifications, including congenital infections, tuberous sclerosis, and prior intrathecal administration of pantopaque contrast media.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




