CHAPTER 17 Upper extremity veins and superior vena cava
Anatomy
Development
In the embryo, the upper limb buds are drained by marginal veins.1 The deep veins develop along with their corresponding arteries. The preaxial and postaxial portions of the marginal vein become the cephalic and basilic veins, respectively.
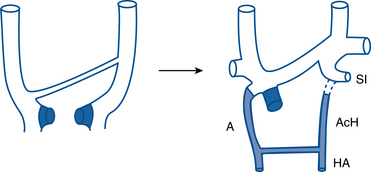
The central thoracic veins are formed from the precardinal (anterior) and common cardinal vessels1 (Fig. 17-1). The upper segment of the precardinal vein becomes the internal jugular vein. The subclavian vein enters from the arm and joins the lower portion of the precardinal vein. An interprecardinal anastomosis connects the two precardinal veins and develops into the left brachiocephalic vein. The lower right precardinal vein becomes the upper superior vena cava. A remnant of the left precardinal vein becomes the left superior intercostal vein.
Normal anatomy
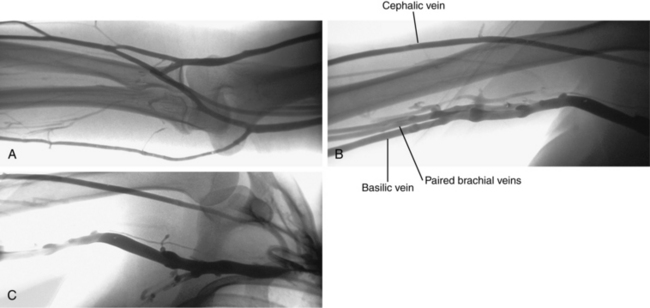
The arms are drained by superficial and deep venous pathways. Numerous perforators connect these systems. Unlike the lower extremities, the superficial system is dominant. It arises from two complex venous plexuses on the dorsal and palmar surfaces of the hand.2 The cephalic and basilic veins originate from the dorsal venous network and run on the radial and ulnar sides of the forearm, respectively. The median vein of the forearm drains the palmar venous plexus and joins the basilic vein near the elbow. The median cubital vein connects the basilic and cephalic veins at the elbow (Fig. 17-2). In the upper arm, the basilic vein lies medial to the biceps muscle and forms the axillary vein at the lateral border of the scapula (see Fig. 17-2). The cephalic vein runs superficial and lateral to the biceps muscle, into the deltopectoral groove, through the infraclavicular fossa, and onto the upper surface of the axillary vein (Fig. 17-3). The deep veins of the forearm follow the radial and ulnar arteries. They unite and then divide at the elbow to form paired brachial veins. These vessels run alongside the brachial artery and then join the basilic vein to become the axillary vein.
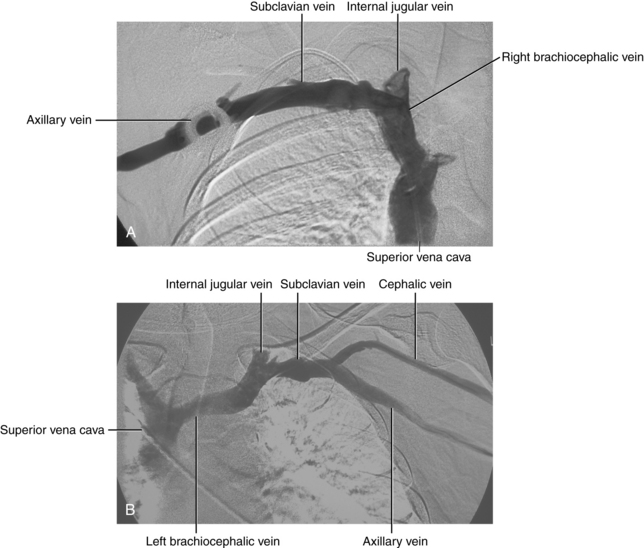
The axillary vein begins at the confluence of the brachial and basilic veins. Several branches of the brachial plexus course between the artery and vein in this region. At the lateral border of the first rib, the axillary vein becomes the subclavian vein (see Fig. 17-3). Several tributaries, including the external jugular vein, enter the subclavian vein. The vein runs in front of the anterior scalene muscle. At the medial edge of the anterior scalene muscle, the internal jugular vein joins the subclavian vein to form the brachiocephalic (innominate) vein.
The right brachiocephalic vein courses almost vertically in front of the right brachiocephalic artery. Tributaries include the right vertebral, internal thoracic (mammary), inferior thyroid, and first posterior intercostal veins. The left brachiocephalic vein (which is more than twice as long as the right) passes inferomedially in front of the left subclavian and left carotid arteries. Branches are comparable to those on the right with the addition of the left superior intercostal and thymic veins. In most cases, the former vessel is the continuation of the accessory hemiazygous vein (see Fig. 17-1). The major lymphatic ducts (including the thoracic duct on the left) enter the venous circulation near the junction of the left subclavian and jugular veins.
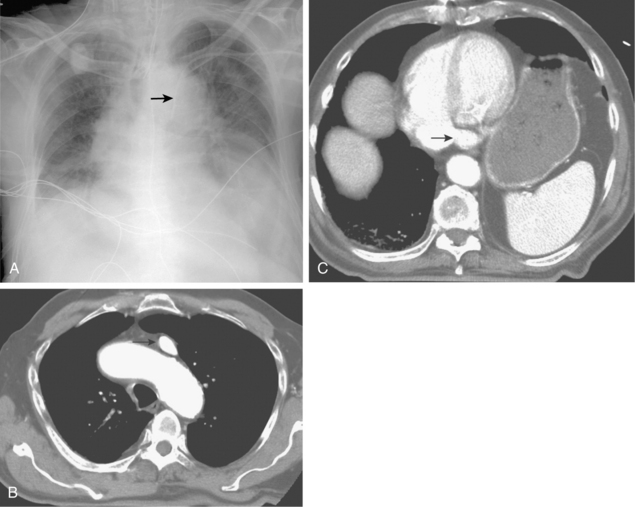
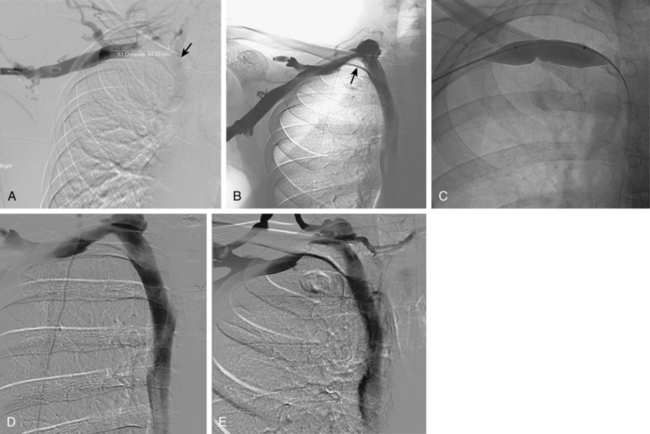
The brachiocephalic veins merge to form the superior vena cava (SVC), which is valveless. The SVC enters the right atrium at about the level of the third costal cartilage. The azygous vein ascends from the abdomen into chest through the right crus of the diaphragm. It arches anteriorly to an opening in the back wall of the SVC above the right main stem bronchus (Fig. 17-4; see also Fig. 17-1). The main trunk of the azygous vein drains the right posterior and superior intercostal veins. The hemiazygous vein ascends to the left of the thoracic aorta. At about the T7-T8 level, it gives off a large branch to the azygous vein and then becomes the accessory hemiazygous vein.
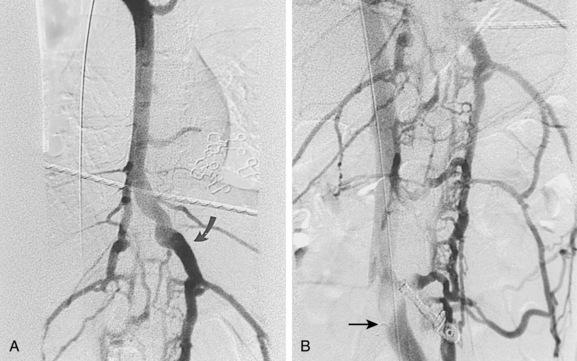
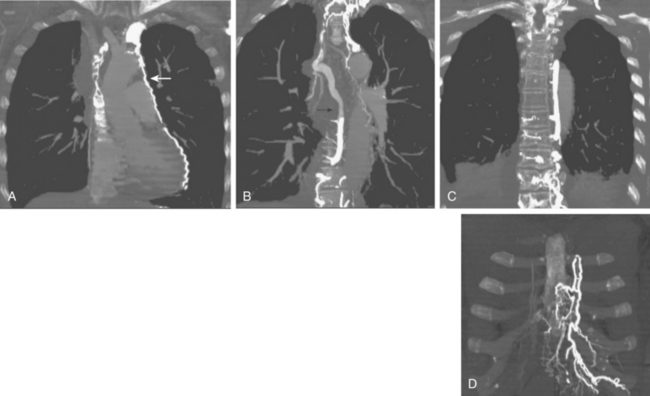
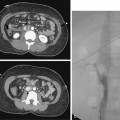
Figure 17-4 Azygous venous system in a patient with a low superior vena cava (SVC) stenosis. A, Injection of the azygous vein directly from the superior vena cava shows retrograde flow down the vein, with filling of the hemiazygous vein (curved arrow) and numerous lumbar collaterals. B, Late phase of the run with imaging over the mid-abdomen shows filling of the left iliac vein (arrow) and inferior vena cava through lumbar and vertebral collaterals.
Valves are present in the superficial and deep veins from the hand to the subclavian trunks. Changes in intrathoracic pressure during inspiration and expiration produce a corresponding increase or decrease in flow through the upper extremity veins, respectively. With rapid inspiration, the internal jugular and subclavian veins may even completely collapse. The Doppler ultrasound flow patterns in the subclavian, internal jugular, and axillary veins reflect both atrial and respiratory activity.
Variant anatomy (online case 105)
The most common anomaly of the upper extremity veins is partial or complete duplication. Rare variants include separate drainage of the brachiocephalic veins into the right atrium and absence of the left brachiocephalic vein with blood return through the left superior intercostal vein.3
Persistent left SVC (duplication of the SVC) is an uncommon anomaly that results from persistence of the left precardinal vein, with or without a maldeveloped left brachiocephalic vein3–5 (Fig. 17-5). It is found in less than 1% of the general population but is more frequent in patients with congenital heart disease. The left-sided moiety usually empties into the coronary sinus.
Isolated left SVC, which is a less common variant, occurs with persistence of the left precardinal vein and regression of the right precardinal vein.6
Variations in azygous and hemiazygous venous anatomy are frequent. In particular, the accessory hemiazygous vein may drain into the azygous, hemiazygous, or left brachiocephalic veins.2
Collateral circulation
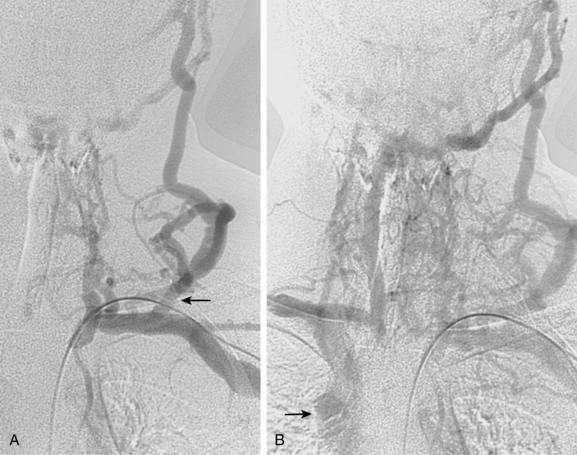
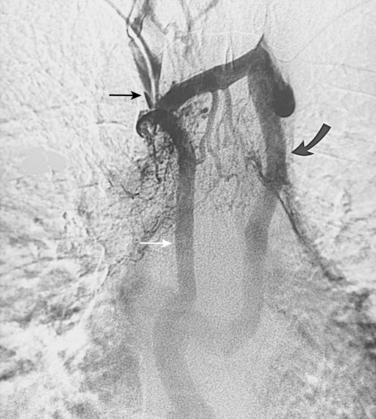
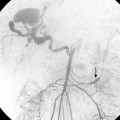
With axillosubclavian vein thrombosis, muscular and superficial veins around the shoulder and thorax are recruited as collateral pathways, which empty into the brachiocephalic, jugular, or azygous veins. With brachiocephalic vein occlusion, the principal collateral pathway is up the ipsilateral jugular vein to the contralateral jugular or brachiocephalic veins via multiple head and neck channels (Fig. 17-6).
With partial or complete obstruction of the SVC, the major collateral routes are through the azygous-hemiazygous system, internal thoracic veins, lateral thoracic veins, and the vertebral venous plexus.7,8 With low SVC obstruction below the azygous insertion, blood flows down the azygous and hemiazygous veins into the lumbar veins and into the iliac veins and inferior vena cava (IVC) (Fig. 17-7; see also Fig. 17-4). With high SVC obstruction above or at the azygous insertion, collateral flow is mainly through right and left superior intercostal branches and the left brachiocephalic vein and then into the azygous system (Fig. 17-8). If the obstruction engulfs the SVC and the brachiocephalic and azygous veins, drainage is through superficial chest wall, lateral thoracic, and internal thoracic veins into the iliac veins and IVC (Fig. 17-9).
Major disorders
Upper extremity venous thrombosis (online case 51)
Etiology
Symptomatic venous thrombosis is much less common in the arm and chest than in the leg.9Box 17-1 outlines the major causes.10–13 In many patients, prior or existing central venous devices of various types are the culprit. Subclavian vein catheters are much more prone to stenosis or occlusion than internal jugular vein lines.10,14 In some populations, stenoses or complete occlusions (often asymptomatic) have been found in up to 50% of individuals with a history of subclavian vein catheters.14–16 Most symptomatic patients have obstruction of the axillary or subclavian veins, with or without more central (brachiocephalic or SVC) occlusion. Hemodialysis catheters are especially problematic. Patients with end-stage kidney disease often require periodic placement of these large-caliber devices. Presence of a maturing or mature dialysis shunt in the arm only exacerbates the problem due to nonphysiologic high blood flow through the outflow veins.
Primary thrombosis (i.e., spontaneous or “effort” thrombosis), also known as Paget-Schroetter disease, accounts for about 20% of cases of upper extremity central venous occlusion.17 In this venous form of the thoracic outlet syndrome, the subclavian or axillary vein is compressed by a musculoskeletal structure, most often between the first rib and subclavius tendon or the costoclavicular ligament. Unlike the subclavian artery, the vein runs in front of the anterior scalene muscle and is not trapped at this site. Chronic intimal injury (often exacerbated by strenuous shoulder activity) combined with slow flow or a hypercoagulable condition can lead to vessel thrombosis.
The natural history of acute upper extremity venous thrombosis is different from the comparable disease in the legs. It was notable in the European RIETE venous registry that co-incident symptomatic pulmonary embolism at presentation was much less common with upper extremity deep vein thrombosis (DVT) (9%) than with lower extremity DVT (29%).9 Pulmonary embolism is ultimately detected in up to 35% of individuals with upper extremity or internal jugular DVT.17–21 Even with anticoagulation, complete recanalization of thrombosed upper extremity veins is not the rule.22 Rather, clots often organize over time, leaving a fibrotic lumen and scarred vein wall. Post-thrombotic syndrome with minor or severe functional disability follows in 7% to 46% of cases.17,23,24
Clinical features
Some patients with acute venous thrombosis are completely asymptomatic. Others complain of arm swelling, pain, cyanosis, coolness, and distended superficial veins. These symptoms can mimic infection, lymphatic obstruction, or blunt trauma. With chronic venous occlusion, arm fatigue after exercise may be experienced. In less than 5% of patients (most of whom have an underlying malignancy), widespread venous thrombosis leads to the syndrome of phlegmasia cerulea dolens and arterial insufficiency.25,26 The typical patient with Paget-Schroetter disease is a young person complaining of the sudden onset of arm swelling and pain. A history of vigorous physical activity (e.g., weight lifting) can often be elicited.
Imaging
Cross-sectional techniques
Color Doppler sonography is the first-line imaging tool for detecting axillosubclavian and central venous stenosis and thrombosis.27,28 Even though ultrasound is simple and safe to perform, the sensitivity and specificity of ultrasound in this setting (82% in one contemporary study) are less than desirable.29 The clavicle and sternum hide most of the central brachiocephalic veins and the SVC from direct visualization. When ultrasound is negative or equivocal and clinical suspicion remains high, catheter or magnetic resonance/computed tomography (MR/CT) venography should be done.
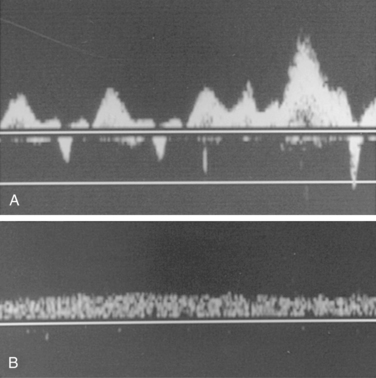
A thorough examination includes study of the accessible portions of the axillary, subclavian, brachiocephalic, and internal jugular veins; peripheral upper arm veins are occasionally interrogated. Color Doppler imaging detects stenoses, occlusions, and collateral channels, and Doppler waveform analysis is added to identify physiologic signs of more central obstruction. Normally, a triphasic atrial waveform with superimposed respiratory variation is present (Fig. 17-10). Absent or monophasic waveforms or asymmetry between sides may be a clue to central obstruction.30 The subclavian vein dilates with a Valsalva maneuver and collapses with rapid inspiration (i.e., sniff test). Indwelling venous catheters do not seem to alter the accuracy of the technique.31
Signs of venous thrombosis include an intraluminal filling defect, absent flow, or an abnormal Doppler tracing (Fig. 17-11). The latter finding can be confirmed with contrast venography. Thrombosis can be missed for several reasons.
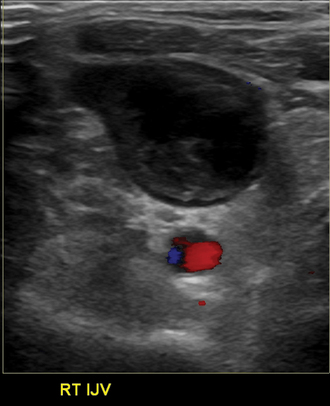
Figure 17-11 Partial thrombosis of the right internal jugular vein is identified by color Doppler sonography.
Because duplex sonography is simple to perform, fairly accurate, and relatively inexpensive, more complex cross-sectional techniques (MR or CT venography) are typically reserved for equivocal cases, for global mapping of the status of upper extremity veins, or for identifying potential vascular access sites in patients with extensive venous disease.32–37
Catheter venography
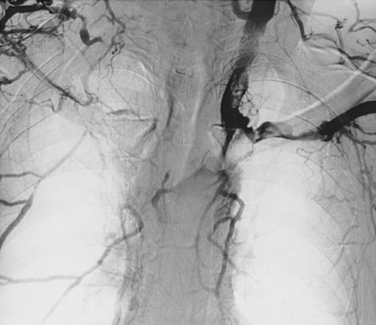
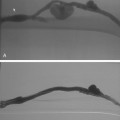
Venography is used to confirm the diagnosis or plan endovascular or surgical treatment of venous thrombosis of the upper extremity. In the acute stage, intraluminal filling defects may be seen (Fig. 17-12). In the subacute or chronic stages, long-segment scarring or stenosis is sometimes evident. Otherwise, the clotted vessels do not opacify and multiple collateral channels fill (see Fig. 17-6). If the brachiocephalic vein or SVC is difficult to visualize from a peripheral arm injection because of slow flow in the arm veins or washout from the internal jugular vein, a catheter should be placed centrally for more direct venography.
Treatment
Medical therapy
The standard first-line therapy for upper extremity venous thrombosis is anticoagulation38 (see treatment of documented pulmonary embolism in Chapter 14). Anticoagulants limit clot propagation and enable recruitment of collateral vessels. Most patients respond well to this approach without further intervention.17 Those with indwelling central venous devices may require catheter removal or long-term anticoagulation.
Endovascular and operative therapy
More aggressive transcatheter interventions are appropriate for:
For acute venous thrombosis (less than 10 to 14 days), enzymatic fibrinolysis with or without adjunctive mechanical thrombectomy devices are favored.38 If a central venous catheter or device is present, it is preferable to remove it. However, patients who are dependent on such catheters may have them left in place. Although systemic infusion can be used, the preferred method is catheter-directed local thrombolysis (see Fig. 17-12) (see Chapter 3 for technical details). Access is usually gained through an ipsilateral basilic or brachial vein. If necessary, the common femoral or internal jugular vein may be used. The patient and operator should understand that complete therapy may require several days of infusion and relatively large doses of fibrinolytic agents. Mechanical thrombectomy devices often serve as adjuncts to chemical lysis.39
Patients are vigorously anticoagulated during the procedure and often require long-term anticoagulation. Underlying stenoses are treated with balloon angioplasty. The role of stents in this setting is controversial.40–42 They are generally avoided in thoracic outlet syndrome, although some reports claim good long-term results after stent placement.43 Stents are used very judiciously in other cases. The drawbacks of stents in the upper extremity and torso include deformity and rethrombosis from extrinsic compression, in-stent restenosis, stent migration, shortening or fracture, and “jailing” of important tributaries such as the internal jugular vein.44
Short-term results of thrombolysis with or without mechanical thrombectomy devices are excellent in about 75% to 85% of cases; thrombolysis is clearly superior to anticoagulation alone for reestablishing flow in fresh occlusions.43,45–47 Late reocclusion is a common problem, particularly when the underlying cause of thrombosis (e.g., indwelling catheter, thrombophilic state, extrinsic compression) is not eliminated. However, the evidence is weak with respect to the long-term benefit of catheter-directed thrombolysis compared with therapeutic anticoagulation for prevention of upper extremity post-thrombotic syndrome.47
For subacute and chronic venous thrombosis (more than 10 to 14 days), primary angioplasty with or without stent placement is recommended (Fig. 17-13
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree