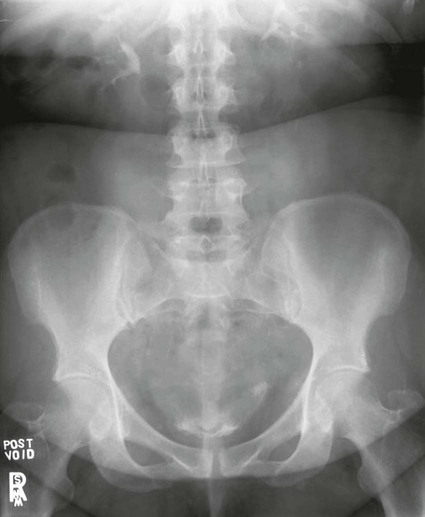
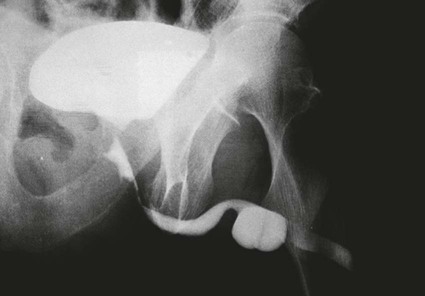
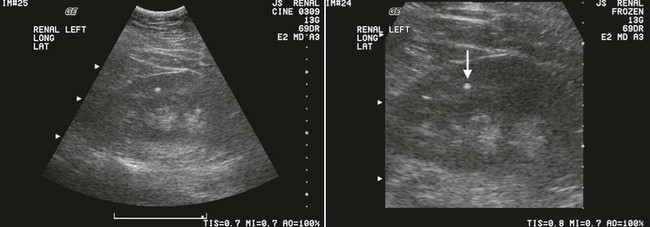
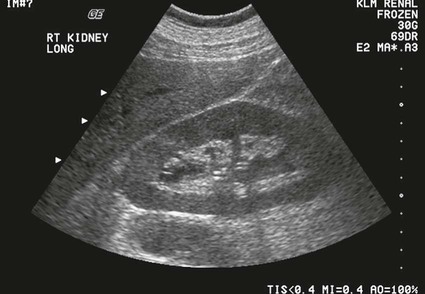
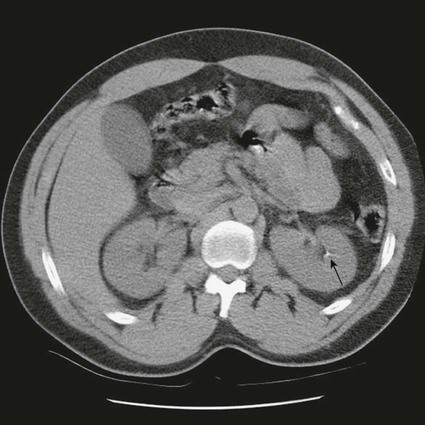
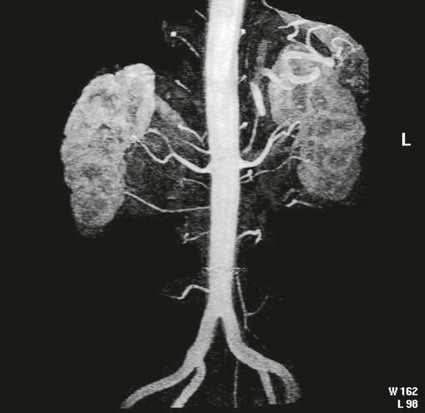
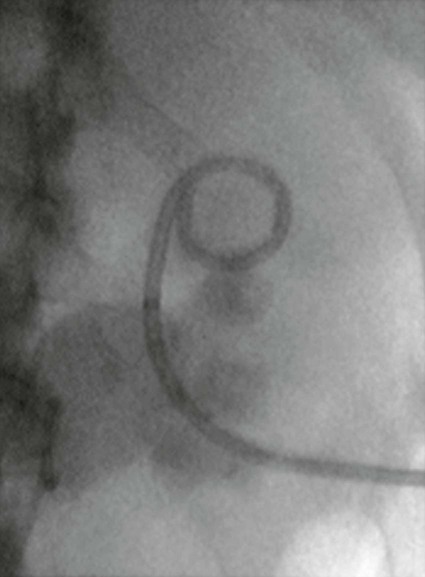
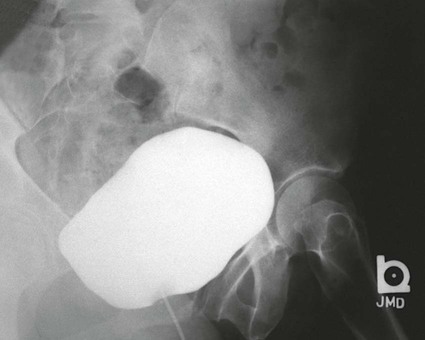
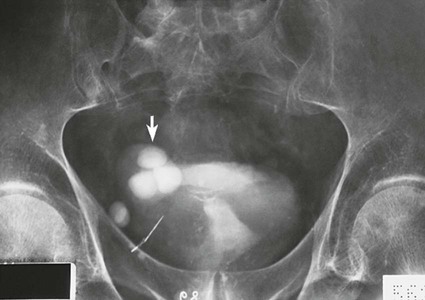
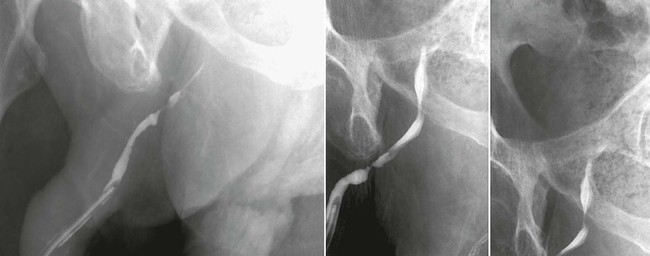
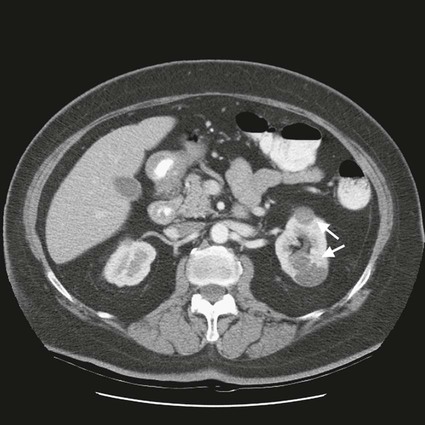
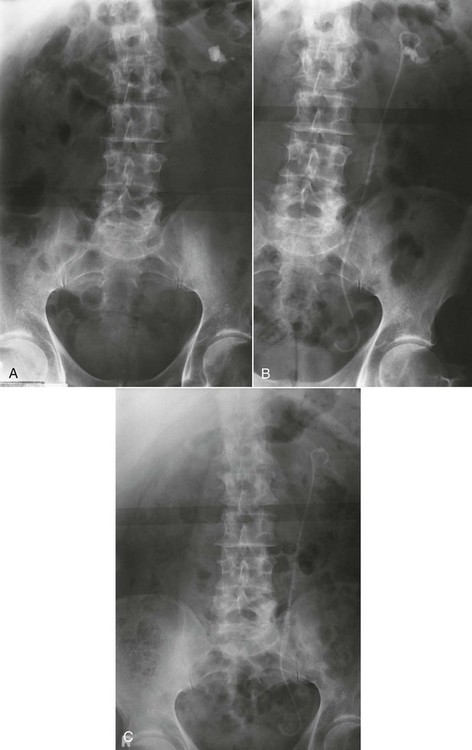
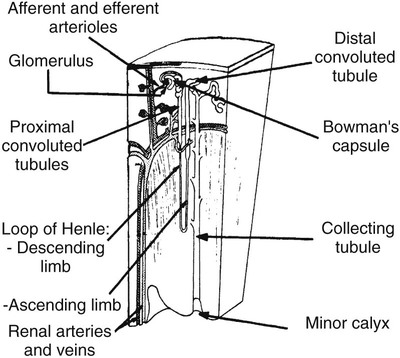
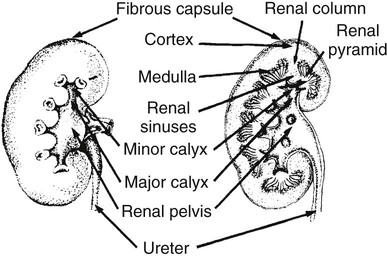
On completion of Chapter 7, the reader should be able to do the following: • Describe the anatomic components of the urinary system and their functions. • Discuss the role of other modalities in imaging the urinary system, particularly sonography and computed tomography. • Discuss common congenital anomalies of the urinary system. • Characterize a given condition as inflammatory or neoplastic. • Identify the pathogenesis of the pathologies cited and the typical treatments for them. • Describe, in general, the radiographic appearance of each of the given pathologies. The urinary system consists of two kidneys, two ureters, a urinary bladder, and a urethra (Fig. 7-1). The urinary system forms urine to remove waste from the bloodstream for excretion. The kidneys are the site where urine is formed and excreted through the remarkable processes of filtration and reabsorption, involving up to 180 liters (L) of blood per day. Urine formed by this process amounts to approximately 1 to 1.5 L per day and passes from the kidneys to the bladder through the ureters. Stored in the bladder, it is eventually excreted through the urethra. The kidneys are retroperitoneal, normally located between the twelfth thoracic vertebra and the third lumbar vertebra. The right kidney lies slightly lower because of the presence of the liver superiorly. The notch located on the medial surface of each kidney is the hilus, the area where structures enter and leave the kidney. These structures include the renal artery and vein, lymphatics, and a nerve plexus. Microscopically, the nephron is the functional unit of the kidney responsible for forming and excreting urine (Fig. 7-2). The nephron unit is composed of the glomerulus, Bowman capsule, and numerous convoluted tubules. Blood flowing through the glomerulus, a ball-like cluster of specialized capillaries, is filtered and cleaned of impurities. Fluid moves out of the glomerulus into Bowman capsules and through the various convoluted tubules, resulting in the production of urine. The nephron unit terminates into a collecting tubule, which forms a tube opening at the renal papilla into a minor calyx. Minor calyces terminate in the major calyces, which, in turn, terminate at the renal pelvis (Fig. 7-3). The ureters extend from the kidneys to the urinary bladder and are approximately 10 inches in length (Fig. 7-4). They normally enter the bladder obliquely in the posterolateral portion of the bladder, equidistant from the urethral orifice in a triangular fashion. A number of variations of this exist. The function of the ureters is to drain the urine from the kidneys to the bladder. KUB (kidney, ureter, bladder) radiography is useful in demonstrating the size and location of the kidneys. These organs may be visible radiographically because of the perirenal fat capsule that surrounds them. The kidneys are generally well fixed to the abdominal wall and are seen to move with respiratory effort. As mentioned earlier, the right kidney is usually located inferior to the left kidney because of the presence of the liver. Men’s kidneys are generally larger than those of women. The kidneys lie in an oblique plane within the abdomen and tend to parallel the borders of the psoas muscle shadows. Evaluation of the kidneys using only a KUB image is limited because the kidney shadows may often be obscured by bowel content and are difficult to visualize because of the inherent low subject contrast in the abdomen. However, KUB radiography is the usual beginning for intravenous urography (IVU), sometimes referred to as intravenous pyelography (IVP) (Fig. 7-5). In this case, its primary purposes are to (1) determine if adequate bowel preparation has been accomplished and (2) visualize radiopaque calculi of the KUB that may otherwise be hidden by the presence of contrast media. The radiologist also examines areas unrelated to the urinary tract because they may hold clues to the diagnosis and may also assist in differentiating between gastrointestinal (GI) and genitourinary disorders. Although the numbers and types of images obtained may vary from one institution to another, a series of collecting system sequence images are the final part of IVU (Fig. 7-6). The renal pelvis, calyces, ureters, and bladder are examined for any abnormalities. The calyces should be evenly distributed and reasonably symmetric. Usually, they appear as buttercup-shaped projections surrounding the renal papillae. Calyceal dilatation may be demonstrated as a result of acute or chronic urinary tract obstruction, obstructive uropathy, or reflux. Dilatation secondary to destruction of the renal pyramids is less common. Because of the peristaltic activity of ureters, only part of their length in a collecting system sequence may be demonstrated (Fig. 7-7). Nonopaque ureteral calculi sometimes cause filling defects and an obstructive dilatation of the ureter. The majority of all urinary tract calculi are found at the vesicoureteral junction. Any pronounced deviation of the ureter suggests the presence of a retroperitoneal mass. Various filling defects may be demonstrated in the contrast agent–filled ureter during IVU, including tumors, blood clots, and nonopaque calculi. Common bladder defects visualized during IVU include urinary catheter balloons, normal uterus and colon, and extrinsic deformities such as uterine or sigmoid colon tumors. A “postvoid” image usually completes an IVU procedure and allows assessment of the bladder function (Fig. 7-8). Cystography is a common radiographic examination for studying the lower urinary tract. This involves insertion of a urinary catheter into the urethra and retrograde filling of the bladder with iodinated water-soluble contrast material (Fig. 7-9). A frequent indication for this procedure is to identify vesicoureteral reflux (VUR). In the normal bladder, increased pressure as the bladder fills effectively shuts down any chance of reflux. Bladder infection, however, may render the ureteral “valve” incompetent, refluxing the infection into the kidney. Cystography may also be used to study congenital bladder anomalies, tumors, diverticula (Fig. 7-10), calculi, bladder rupture, or neurogenic bladder. Voiding (micturition) cystography is sometimes used in conjunction with retrograde cystography to allow study of the urethra on voiding. Urethrography may be accomplished using the antegrade approach, as with voiding cystourethrography, or retrograde when cystography is not necessary. The antegrade approach is used to study the posterior urethra, especially in the male patient, and the retrograde approach is helpful in studying the anterior urethra (Fig. 7-11). The usual intent of voiding cystography is to allow study of a urethral stricture (Fig. 7-12). Sonography is a noninvasive method of imaging both functioning and nonfunctioning kidneys. Because sonography can clearly demonstrate the parenchymal structure of the kidney and the renal pelvis without the use of contrast agents, it is becoming the primary method of visualizing the kidneys and evaluating most renal disorders. It is useful in evaluating kidney stones (Fig. 7-13), calcifications, hydronephrosis (Fig. 7-14), abscesses, renal masses, and renal cysts and to assess renal size, atrophy, or both. Sonography is the modality of choice for evaluating individuals after kidney transplantation. Doppler techniques are helpful in assessing blood flow in the renal arteries and veins for both transplant recipients and individuals with suspected renal artery stenosis. Sonography is also used to visualize abnormalities of the urinary system present in the fetus. CT is an excellent modality for imaging the kidneys because it can detect small differences in tissue densities within the body. Kidneys can be visualized on CT with or without the use of a contrast agent. Abdominal CT is particularly important in determining the nature of renal masses, either solid or cystic, which may not be visible on a KUB radiograph because of the presence of gas in the bowel. CT evaluation of the urinary system generally requires the use of an IV contrast agent to differentiate renal cysts from solid masses and to evaluate the extent of the lesion (Fig. 7-15). Because most institutions use an automatic injector in CT, scanning may begin when the bolus of contrast medium is injected or shortly after injection, and a delay is programmed into the scanner to allow the contrast medium to reach the bladder before the pelvis is imaged. CT is also useful for looking for sites of obstruction caused by renal calculi or retroperitoneal masses, which may distort the urinary tract; assessing renal infection or trauma; and staging tumors of the lymph nodes. A CT renal stone study is considered the imaging modality of choice by the American College of Radiology (ACR) when patients present with an acute onset of flank pain or other symptoms suggest the presence of renal calculi. Because CT displays excellent contrast resolution, stones are identified more easily than with conventional radiography, but without the use of an intravenous contrast agent (Fig. 7-16). In addition, pelvic CT is the imaging modality of choice for the evaluation of bladder tumors or masses. The role of magnetic resonance imaging (MRI) has greatly improved as a result of breath-hold imaging sequences and bolus injections of gadolinium contrast agents. Abdominal MRI is useful in follow-up studies in patients with known renal cell carcinoma or invasive bladder cancers and adrenal masses. Additionally, magnetic resonance angiography (MRA) is now highly recommended by the ACR in the diagnosis of renovascular hypertension (Fig. 7-17). Contrast-enhanced three-dimensional MRA obtains coronal images of the renal arteries in as little as 20 seconds. The images can then be rotated for better visualization. MRA is also an excellent modality for demonstrating other vascular anomalies such as thrombosis, aneurysms, and arteriovenous malformations (AVMs). Because it allows for imaging of the urinary system in all three planes, it is also used in conjunction with CT for the evaluation of renal masses and their extensions. In cases of renal cyst evaluation, MRI is capable of differentiating between fluid accumulation from hemorrhage and infection. Pelvic MRI is used to readily demonstrate the seminal vesicles and prostate gland in men as well as masses within the urinary bladder. Because of its ability to clearly image soft tissue, pelvic MRI allows thorough evaluation of invasive cancers within the urinary bladder. Percutaneous nephrostography is an antegrade study in which the contrast medium is injected directly into the renal pelvis. It involves posterolateral insertion of a needle or catheter into the renal pelvis using medical sonography, fluoroscopy, or sometimes a combination of both modalities (Fig. 7-18). The nephrostomy tube may be left in place to provide drainage of an obstructed kidney or to allow retrieval of the calculus with a basket catheter. Sometimes the procedure is used to relieve obstruction in patients for whom immediate surgery is not possible. Extracorporeal shock wave lithotripsy (SWL) is a method used to locate and treat renal calculi. After the location of the stone is determined radiographically, fluoroscopy or sonography aids in alignment of a high-frequency shock wave directed at the stone. If the treatment is successful, the stone disintegrates into fragments and is excreted via urination, thus helping the patient avoid surgery and a much lengthier recovery period (Fig. 7-19).
Urinary System
Anatomy and Physiology
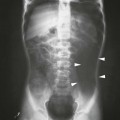
KUB Radiography
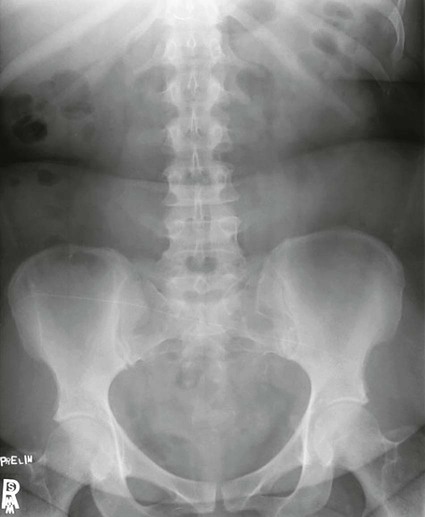
Intravenous Urography
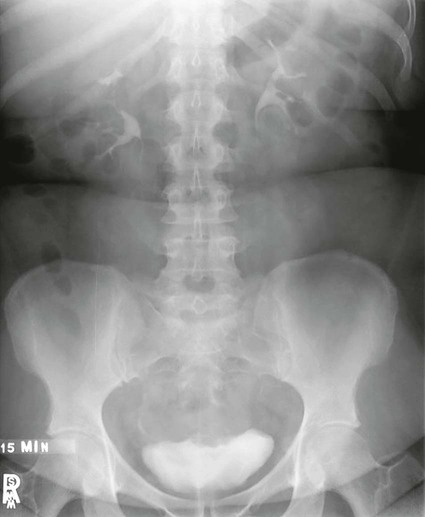
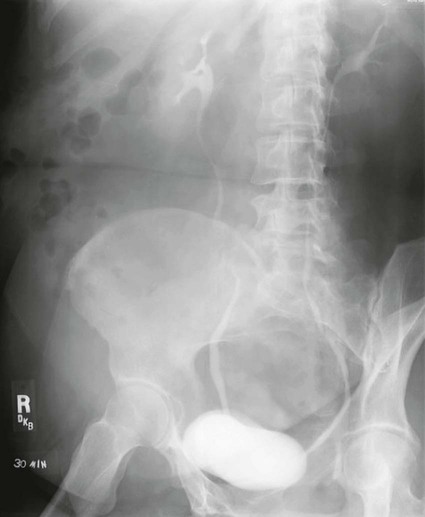
Cystography
Sonography
Computed Tomography
Magnetic Resonance Imaging
Interventional Procedures and Techniques
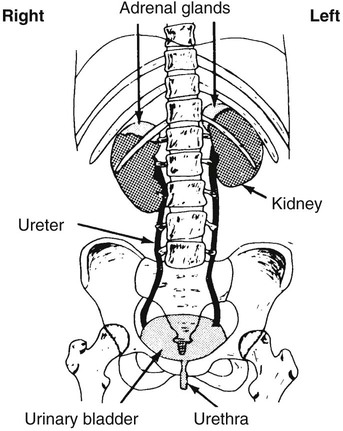
Urinary System

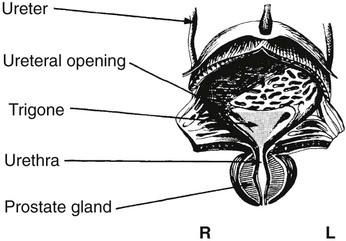
 inches in length, whereas the male urethra is approximately 8 inches in length. In men, the urethra passes through the prostate gland and also serves as a part of the reproductive system by receiving seminal fluid via the ejaculatory ducts, which open into the urethra from the prostate. The male urethra is classified by three separate portions: (1) the prostatic portion, (2) the membranous portion, and (3) the cavernous portion. The urethra opens to the exterior of the body via the urinary meatus.
inches in length, whereas the male urethra is approximately 8 inches in length. In men, the urethra passes through the prostate gland and also serves as a part of the reproductive system by receiving seminal fluid via the ejaculatory ducts, which open into the urethra from the prostate. The male urethra is classified by three separate portions: (1) the prostatic portion, (2) the membranous portion, and (3) the cavernous portion. The urethra opens to the exterior of the body via the urinary meatus.