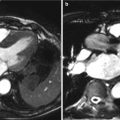
Fig. 27.1
Reconstructing a double oblique transverse image of the basal ring from an MDCT in an 82-year-old male patient with severe symptomatic AS. From the coronal projection (a), a vertically oriented oblique tool is placed to produce a sagittal oblique reconstruction of the ascending aorta. A transverse or axial cut plane is then placed on the sagittal reconstruction (b) at the level of the commissures. This transverse cut plane yields a double oblique transverse image of the aortic root (c). The dataset is then scrolled up or down until the most caudal attachments of the aortic valve are identified (d). The nadirs of all three cusps must be identified on the one transverse image, ensuring the appropriate plane for assessment of the aortic annulus. Not uncommonly, these simple four steps yield only a double oblique transverse image of two of the aortic cusps, and it may often require the further rotation of the sagittal oblique projection until the third cusp comes into view
Although much was known about the anatomical configuration of the annulus, insight into the in vivo anatomy of the aortic valvular complex—previously available only from surgical or pathological observation of a non-beating heart—only became possible with modern three-dimensional imaging techniques [2]. The aortic annulus has been shown to be noncircular on both 3D transesophageal echocardiography (3D TEE) and multidetector computed tomography (MDCT) [3–5]. As a result of this elliptical configuration, the aortic annulus does not lend itself to accurate evaluation by a two-dimensional imaging modality that measures an axial diameter.
The aortic root is dynamic, with the aortic annulus subject to pulsatile changes of the cardiac cycle and contour deformity related to movement of the aorto-mitral continuity and dynamic left atrial volume and pressure changes [6, 7]. Subsequent changes in geometry and dimensions have to be considered when selecting a THV size and historically have been underappreciated by two-dimensional imaging.
Aortic Annulus Measurement with Computed Tomography
Enabled by the isotropic voxels acquired with CT, the aortic annulus can be assessed in its true plane at the level of the basal ring. When the plane of the aortic annulus is achieved, it can be measured in a number of ways. Most commonly, the long axis (maximum) and the short axis (minimum) are measured allowing for the calculation of a mean diameter. Planimetry can also be performed enabling both perimeter and area measurements (Fig. 27.2). Derived diameters can then be calculated based on the formula for the area of a circle or the perimeter of a circle, respectively, although there are real limitations in deriving diameters from these three-dimensional measurements [8, 9].
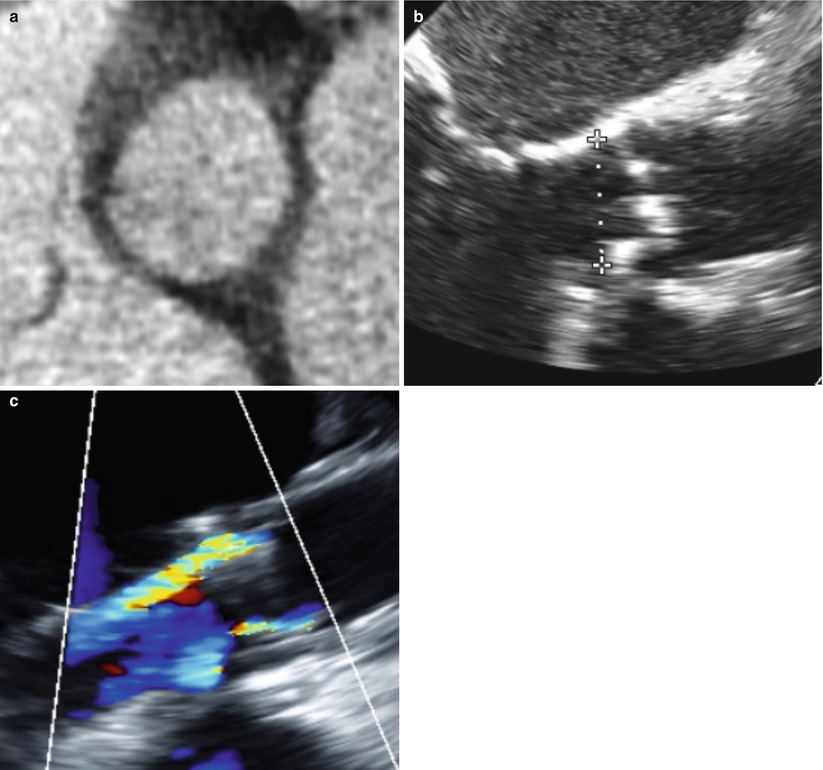

Fig. 27.2
Representative images of the basal ring in a 78-year-old male patient with aortic stenosis. The annulus was deemed too large for a balloon-expandable prosthesis with a mean diameter of 30.7 mm (short axis of 27.9 mm and long axis of 33.5 mm) (a) and an area of 8.04 cm2 (b). The 29-mm SAPIEN XT has a nominal area of 6.6 cm2 which is clearly too small for this annulus and would result in approximately 17 % undersizing of the annulus. The post-TAVR TTE shows the resultant moderate paravalvular regurgitation (c)
The above CT measurements have been shown to be highly reproducible with low inter- and intra-observer variability [6, 8]. The area-derived diameter and basal ring average diameters are consistently measured as larger than the corresponding echocardiographic measurements, with a mean difference when compared with TEE-derived diameter of 1.5 ± 1.6 and 1.1 ± 1.7 mm respectively.
Aortic Annular Geometry and THV Sizing: Evolving Role of CT
Noninvasive assessment and measurement of the aortic annulus is essential for the success of TAVR. An undersized valve increases the risk of post-procedural paravalvular aortic regurgitation, while excessive oversizing may increase the risk of coronary occlusion or annular rupture. Excessive oversizing may also result in embolization, as the valve may not capture appropriately resulting in the so-called melon seeding and subsequent embolization into the aorta particularly in the setting of significant septal hypertrophy. Reproducible and accurate measurements of the aortic annulus allow appropriate selection of THV size (Box 27.1). Unlike surgical aortic valve replacement, where prosthesis sizing is performed using a sizing probe under direct visualization, TAVR relies exclusively on pre- and periprocedural imaging for valve selection. Annular measurements have historically been performed using two-dimensional (2D) transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), or calibrated aortic angiography, with discordance between these measurements being common [9, 10]. The noncircular geometry of the aortic annulus results in an underappreciation of annular size when using two-dimensional imaging techniques [6–8]. In a recent analysis performed in 125 patients undergoing TAVR from two sites, the mean difference between the long and short axis of the aortic annulus was 5.5 mm [6].
There has been growing interest in better defining the shape and size of the annulus through the integration of three-dimensional (3D) imaging tools such as CT, magnetic resonance imaging (MRI), and 3D TEE [3]. Annular assessment with 3D TEE has reported larger annular sizes than those observed using traditional 2D TEE [3]. ECG-gated CT was historically performed in patients before TAVR implantation to evaluate peripheral vascular access, annular plane, and coronary height, however, is increasingly being used to measure annular size [4]. Current sizing recommendations and clinical practice are derived from the currently available randomized data [9, 10] in which the annular size was estimated using echocardiographic measurements. Clinical outcomes with TEE-based annulus determination and routine oversizing of the valve by 1 to 2 mm have been good, but the burden of post-TAVR moderate or severe paravalvular aortic regurgitation (PAR) remains approximately 10–15 % [11]. The importance of post-TAVR PAR is increasingly recognized as being of great importance with the recently published 2-year PARTNER B outcome data not only suggesting that 40 % of patients alive at 2 years have at least mild PAR, but, perhaps more importantly, that those patients with PAR have a significantly worse prognosis than those without [11]. While PAR may not be exclusively related to undersizing, there is a growing awareness that two-dimensional imaging does not allow for an adequate appreciation of annular geometry and may lead to inappropriate sizing. This issue has led to the exploration of three-dimensional imaging modalities for annular sizing, such as 3D TEE and CT. The hope is that annular measurements acquired using 3D techniques will provide a more global assessment of annular geometry and eccentricity, allow for more accurate sizing of the THV, and thereby reduce the burden of post-TAVR PAR [6, 12]. At present, there are specific annular size limitations for TAVR based on echocardiographic measures of the annulus. For the Edwards SAPIEN valve, the annulus must measure between 18 and 25 mm; for the current generation of CoreValve, the annulus must range between 20 and 27 mm. Recently, both a 20-mm transfemoral and a 29-mm transfemoral/transapical balloon-expandable SAPIEN XT valve have become available.
Box 27.1 Methods of CT Aortic Annular Sizing
Measure | Recommendation | Used clinically? |
|---|---|---|
Area | Yes | Currently used at certain centers for balloon-expandable valve |
Coronal measure | No | Obliquity of aorta different for different individuals |
Long-axis diameter | No | Excess variability |
Mean diameter | Yes | Currently used at certain centers for balloon expandable valve |
Max diameter – stent diameter >4 mm | Yes | Currently used at certain centers for balloon expandable valve |
Perimeter | Yes | Currently used a measure for self-expanding valves |
Sagittal | No | Obliquity of aorta different for different individuals |
Short axis diameter | No | Excess variability |
Proposed CT Sizing Guidelines for Transcatheter Heart Valves
While sizing guidelines with CT remain a goal for TAVR, it must first be recognized that the implications of three-dimensional sizing are different for balloon-expandable and self-expanding prostheses. For the balloon-expandable prosthesis, a CT-based THV sizing scale is a work in progress. This process has largely grown out of retrospective analyses of cohorts with and without PAR in an attempt to better understand appropriate THV sizing. Gurvitch et al. [8] have shown that CT sizing using the mean of basal short- and long-axis diameters could influence or modify TAVR strategy in up to 44 % of patients. Given the success of the procedure, the suggestion of altering sizing in 40–45 % of cases has largely been rejected by the TAVR community. This is almost certainly appropriate given that at present the only randomized data documenting the efficacy of TAVR is based on echocardiographic sizing of the annulus. However, when the THV sizing cutoffs were adjusted to allow for the measured differences in diameters between echocardiographic-derived and CT-derived data, the THV size would have been modified in only 10–12 %.
While the evidence does not yet support the replacement of standard echocardiographic annular measurement with three-dimensional techniques, the analysis of the aortic annulus in its true plane with bidimensional and area-based measurements already provides useful adjunctive data to two-dimensional echocardiographic assessment. While these measurements have not been validated or integrated in randomized trials, they have already proven to be helpful in predicting complications of THV undersizing such as paravalvular leak [6, 12].
For CT measures of the aortic annulus to move beyond interesting additive information, a CT-based sizing scale must be integrated into THV selection. We propose a sizing scale based both on the mean of the short and long axis of the basal ring and the area measurement of the basal ring. In order to minimize the risk of significant PAR, the implanted THV size should be greater than the three-dimensional area of the annulus on CT. In a recent analysis of our cohort, for THVs that were oversized by at least 10 % of the annular area, there were no cases of significant PAR [6]. We recommend making these measurements in end systole comparable to echocardiographic measurements [6] as the area and mean diameter are larger in this phase of the cardiac cycle, although we recognize that the data is evolving and that perimeter measurements may in fact be more stable throughout the cardiac cycle [13]. It has been suggested that sizing based on diastolic measurements may modify sizing in up to 20–25 % of cases depending on the sizing thresholds used [14]. We do note that intentional oversizing may come with a potential cost of coronary occlusion or annular rupture, but this has not been our experience with a historical unintentional oversizing of the area of the basal ring by approximately 5 % over the last 125 cases on the basis of echocardiographic based sizing [14].
Additionally, the fear that CT sizing will result in a greater degree of oversizing may not be well founded, as the limitations of two-dimensional echocardiographic sizing have led to a great deal of variation in annular oversizing with unintentional oversizing of the annular area by as much as 65.6 % and undersizing by as much as 22.8 % [14]. Importantly, there were no cases of coronary occlusion or of annular injury in the cohort analyzed. In addition, in the cases where the prosthesis is oversized, the THVs remain fully expanded, with under expansion only noted once extreme levels of oversizing, in the magnitude of 40–50 %, are reached. Jilawahi and colleagues [12] recently documented similar findings that three dimensionally derived cross-sectional measurements of the aortic annulus were superior to conventional two-dimensional echocardiographic sizing in the prediction of patients with significant PAR. Interestingly, CT cross-sectional assessment of the aortic annulus was reported to affect device sizing and patient selection and to somewhat reduce post-TAVR PAR. Similar to past assessments, CT measurements were reproducible and could be precisely defined with retrospective ECG-gated acquisitions. While this data is quite compelling, the CT sizing cohort was quite small, and the authors do not provide significant details for the integration of CT measures for sizing nor a specific CT sizing scale.
Proposed CT Sizing Guidelines for Balloon-Expandable Valves
Our group has proposed a different sizing guideline (Table 27.1). The recommendation is to select a prosthesis with a cross-sectional area modestly larger than the CT-derived aortic annulus area. A target of 10–15 % area oversizing with upper and lower limits of 20 to 0 % is suggested (Fig. 27.3). The Vancouver CT sizing guidelines target this range of oversizing while being mindful that fully deploying currently available balloon-expandable prosthesis sizes (20, 23, 26, and 29 mm) (Fig. 27.4) would necessitate accepting a broader range of oversizing than is desirable. Since diameter sizing either by direct measurement or through derivation from an area measurement can only provide modest accuracy and reproducibility, we avoided this approach [7].
Table 27.1




The Vancouver CT sizing guidelines. The recommendation is to select a prosthesis with a cross-sectional area modestly larger than the aortic annulus. A target of 10–15 % area oversizing with upper and lower limits of 1–20 % is suggested
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree