Use of PET and PET/CT in the Evaluation of Patients with Head and Neck Cancer
Todd M. Blodgett
Alexander Ryan
Barton Branstetter IV
Protocols for Evaluation of Head and Neck Cancer
Combined positron emission tomography (PET) and computed tomography (CT) has several advantages over either CT or PET alone in the evaluation of patients with head and neck cancer. The accurate coregistration of images and improved localization achieved with a combined modality are particularly important in the neck, where there is not only anatomical complexity, but several structures that demonstrate variable physiologic fluorodeoxyglucose (FDG) uptake.
In order to maximize the utility of PET/CT, it is important to optimize the several scan protocol options available when performing PET/CT for the evaluation of patients with head and neck cancer. A typical baseline diagnostic PET/CT for a patient with head and neck cancer is increasingly common to include a full-power CT scan with intravenous contrast in addition to an FDG PET scan acquired during the same imaging session on a hardware combined PET/CT device. Oral contrast may or may not be utilized, depending on the extent of area being scanned. A typical protocol for patients with head and neck cancer might include the skull base through the abdomen; therefore, oral contrast is not routinely utilized in the authors’ protocol, but oral contrast is routinely utilized in some centers. FDG must be injected at a consistent time interval prior to the start of patient imaging; a relatively standard protocol would involve injection of FDG 1 hour prior to the commencement of the scan. Longer times from injection until imaging are used by some groups as the tumor/background uptake ratios typically rise with time postinjection; however, about 60 minutes postinjection of tracer is very common to commence imaging.
Neck immobilization is an important protocol consideration with the evaluation of patients with head and neck cancer, as this is the area of interest. A variety of soft collars and external immobilization devices are available to help minimize or eliminate neck movement.
Arm positioning for the examination is also an important consideration. The two most commonly used positions for arm positioning during PET/CT imaging are standard “arms up” and “arms down.” In general, the most important determinant of arm positioning in PET/CT is the indication of malignancy that is being evaluated and subsequent areas of interest being scanned. The goal of any arm positioning protocol should be to minimize CT beam-hardening artifacts in the area of interest. Therefore, for patients with head and neck cancer it is usually preferable to scan patients with arms down, at least at the level of the neck. Alternatively, two scans may be performed, one with data acquisition through the chest and abdomen (with or without the pelvis) while the arms are positioned up followed by a more limited second scan acquisition through the neck area with arms positioned down. Care should be taken to ensure adequate overlap of scans at the thoracic inlet, as lower neck structures can shift substantially with the change in arm position.
The most important CT consideration for patients with head and neck cancer is whether, when, and how to use intravenous contrast and whether to use low- or high-dose CT. Because of the anatomical complexity of the neck, if logistically possible, a diagnostic CT with contrast is typically recommended. This will make it easier to differentiate the vascular from nonvascular structures, as well as to distinguish pathology from normal structures more clearly. Vascular structures can contain FDG, and such uptake can sometimes be confused with small nodes in noncontrast studies.
Other options include performing a low-dose, noncontrast CT (40 to 80 mA), which reduces radiation exposure to the patient but may have a lower sensitivity and specificity for the detection of malignancy on its own, although this has not been rigorously studied in a variety of clinical settings. It can be used for simple localization purposes, but is typically more difficult to interpret due to the lack of structural differentiation. The quality of the scan is too poor
to charge for reimbursement in most centers for the PET alone. However, since most of the tumor detection is being done with the FDG, the quality of the CT scan may not be as critical to the total diagnostic accuracy as the PET plus the localizing information from the CT. It is possible that for follow-up PET studies, such as those used for tumor surveillance, that there is little benefit from the use of a full-contrast CT scan. Not all noncontrast CT scans are performed the same way. Full-dose, noncontrast CT is another option that may be the best for patients with a significant contrast allergy.
to charge for reimbursement in most centers for the PET alone. However, since most of the tumor detection is being done with the FDG, the quality of the CT scan may not be as critical to the total diagnostic accuracy as the PET plus the localizing information from the CT. It is possible that for follow-up PET studies, such as those used for tumor surveillance, that there is little benefit from the use of a full-contrast CT scan. Not all noncontrast CT scans are performed the same way. Full-dose, noncontrast CT is another option that may be the best for patients with a significant contrast allergy.
Contrast-enhanced CT offers the most advantages to the interpreting physician, but it does require medical personnel immediately available to respond to unexpected contrast reactions. One potential disadvantage of using contrast in PET/CT imaging is the presence of attenuation-correction artifacts that are unique to PET/CT. These are discussed in detail at the end of this chapter. Although attenuation-correction artifacts generally are easy to recognize and are a valid reason to avoid intravenous contrast for qualitative PET assessments, the interpreting physician must be aware of these artifacts in order to avoid confusion with malignancy. Further, there can be some modest alterations in apparent standardized uptake value (SUV) in the presence of high concentrations of intravenous contrast.
Another approach involves a low-dose noncontrast CT prior to PET imaging (for the purpose of artifact-free attenuation correction) followed by a full-dose contrast CT of diagnostic quality. This is ideal from an imaging point of view but exposes the patient to potentially unnecessary radiation. However, this approach may be acceptable if contrast CT is only needed for a small area of the patient such as the head and neck.
Optimizing arm positioning and intravenous contrast into a single algorithm is a convenient approach. A full CT scan algorithm might include a noncontrast low-dose CT with arms up performed first (this CT will be utilized for attenuation correction) followed by CT performed with contrast after the PET acquisition. During the contrast-enhanced CT acquisition, the arms would be positioned at the side to minimize beam hardening through the area of interest. Another approach is to perform the neck study first with no contrast and the arms down, followed by the CT with contrast. This practice varies somewhat by center, but it is suggested that the use of intravenous contrast be seriously considered in any protocol designed to detect cancer in the head and neck region.
It is not clear whether all patients with a history of head and neck cancer will benefit from a diagnostic CT as part of their PET/CT workup. In general, when patients are suspected of having active disease during their initial evaluation, during therapy, and in the interim following therapy, contrast-enhanced CT is recommended. An exception can be made for patients who have had a contrast-enhanced CT performed recently. Asymptomatic patients with successfully treated malignancies undergoing surveillance with low suspicion of viable cancer and those with a recent diagnostic CT are good candidates for low-dose CT as part of their PET/CT examination.
Physiologic Fluorodeoxyglucose Distribution in Head and Neck
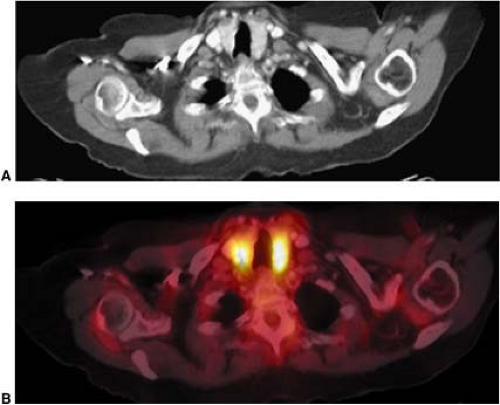
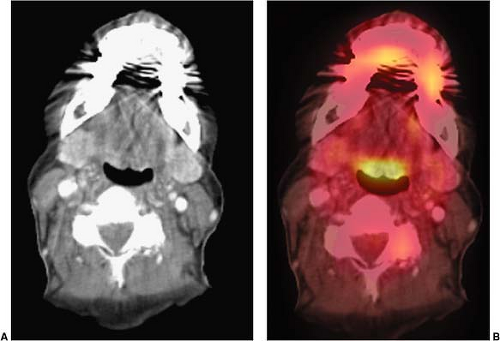
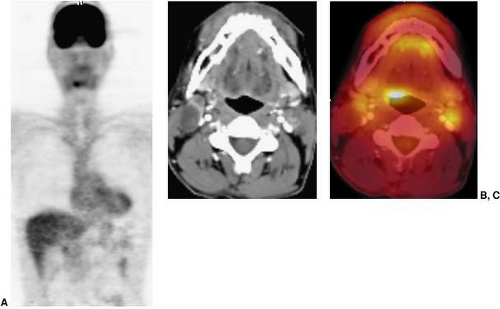
Several normal structures in the head and neck may demonstrate physiological FDG activity on PET or PET/CT. It is important to be familiar with the appearance of normal, as well as atypical, patterns of physiological FDG uptake. Among the structures that take up FDG normally are the salivary glands, thyroid gland, brown fat, lymphoid tissue, mucosal tissue, and muscles of the neck, pharynx, and face. FDG is taken up by salivary glands and excreted into the saliva to a limited extent (1). The parotid, submandibular, and thyroid glands can demonstrate mild to intense symmetric uptake (Figs. 8.7.1 and 8.7.2).
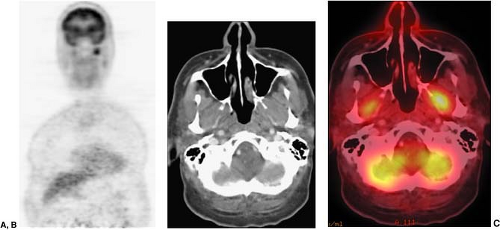
Brown fat may be found throughout the neck and thorax as well as outside the neck (Fig. 8.7.3) (2,3,4). FDG is taken up by macrophages and lymphocytes, therefore, lymphoid structures are also commonly appreciated on FDG PET scans (Fig. 8.7.4). Examples of lymphoid structures include all the structures of Waldeyer’s ring (adenoids, palatine, and lingual tonsils), as well as lymph nodes (5,6,7,8). Mucosal tissue such as that found in the nasal turbinates and seen throughout the oro- and nasopharynx, commonly shows FDG uptake. Muscles are among the many normal structures commonly seen on FDG PET or PET/CT studies (Fig. 8.7.5). Muscles of mastication, including the pterygoid muscles, and muscles of the oral floor may also be seen (Fig. 8.7.6), as well as the pharyngeal constrictor muscles, muscles controlling the vocal cords, and the vocal cords themselves (Fig. 8.7.7) (9). An in-depth review of the more atypical physiologic FDG uptake patterns and pitfalls is provided at the end of this chapter.
Brown fat may be found throughout the neck and thorax as well as outside the neck (Fig. 8.7.3) (2,3,4). FDG is taken up by macrophages and lymphocytes, therefore, lymphoid structures are also commonly appreciated on FDG PET scans (Fig. 8.7.4). Examples of lymphoid structures include all the structures of Waldeyer’s ring (adenoids, palatine, and lingual tonsils), as well as lymph nodes (5,6,7,8). Mucosal tissue such as that found in the nasal turbinates and seen throughout the oro- and nasopharynx, commonly shows FDG uptake. Muscles are among the many normal structures commonly seen on FDG PET or PET/CT studies (Fig. 8.7.5). Muscles of mastication, including the pterygoid muscles, and muscles of the oral floor may also be seen (Fig. 8.7.6), as well as the pharyngeal constrictor muscles, muscles controlling the vocal cords, and the vocal cords themselves (Fig. 8.7.7) (9). An in-depth review of the more atypical physiologic FDG uptake patterns and pitfalls is provided at the end of this chapter.
Conventional Imaging Modalities
Conventional Imaging
One of the reasons that conventional imaging suffers when assessing nodal metastases is that the CT and magnetic resonance imaging (MRI) characteristics of cancer do not differ sufficiently from one
another to offer a truly specific diagnosis. The lack of reliable criteria for identifying metastatic cervical lymph nodes based on size is understandable given the limitations of the techniques applied (10). Size criteria are most commonly reported, but experts cannot even agree on which axis (short or long) is a better determinant of malignancy. Although much of the literature reports use of the short axis, the long axis may correlate better with clinical assessments of the nodes. Different thresholds for lymph node size have been studied, ranging from 5 to 15 mm. As expected, smaller thresholds result in higher sensitivities at the expense of specificity. No single size criterion is sufficient, however, because nodes less than 5 mm can contain foci of malignancy. Similarly, large nodes may be reactive and not contain cancer. Size alone is simply not reliable for detecting cancer in lymph nodes.
another to offer a truly specific diagnosis. The lack of reliable criteria for identifying metastatic cervical lymph nodes based on size is understandable given the limitations of the techniques applied (10). Size criteria are most commonly reported, but experts cannot even agree on which axis (short or long) is a better determinant of malignancy. Although much of the literature reports use of the short axis, the long axis may correlate better with clinical assessments of the nodes. Different thresholds for lymph node size have been studied, ranging from 5 to 15 mm. As expected, smaller thresholds result in higher sensitivities at the expense of specificity. No single size criterion is sufficient, however, because nodes less than 5 mm can contain foci of malignancy. Similarly, large nodes may be reactive and not contain cancer. Size alone is simply not reliable for detecting cancer in lymph nodes.
Other criteria for malignancy have recently gained favor. The presence of central necrosis is almost always indicative of malignancy in adult patients. Caution should be taken, however, that a central fatty hilum (generally more indicative of benignancy) is not confused with central necrosis (indicative of malignancy). It should also be recognized that some infectious processes can also cause central necrosis of lymph nodes.
Contrast enhancement characteristics are also important. Lymph nodes that enhance earlier and to a greater degree than their counterparts are more likely to contain tumor. The border of a suspicious node may be helpful; an ill-defined border suggests extracapsular spread. Of course, most metastatic nodes will not have progressed to extracapsular extension, so a well-defined border cannot be taken as a sign of benignancy.
Perhaps the best anatomic characteristic for distinguishing benign from malignant nodes is the configuration of the node. Reniform nodes, especially those with an identifiable hilum, are almost always benign. Spherical nodes are more worrisome.
The decision of whether to use CT or MRI for the initial evaluation of a head and neck tumor depends on the referring physician and radiologist’s personal preference. Some feel that MRI defines tumor margins better than CT, but others feel that high-resolution CT has bridged the diagnostic gap, so the added expense and inconvenience of MRI is not warranted. Clinical studies show CT to have a small but measurable advantage in the assessment of cervical lymphadenopathy (10).
In a few anatomic areas, the choice between CT and MRI is clearer. Tumors of the skull base and temporal bone are usually first imaged with CT to establish bony extent. MRI is often called on secondarily in these cases. Tumors of the oral tongue are better evaluated with MRI. MRI is far superior for establishing the presence or absence of perineural spread.
Advanced Cross-sectional Techniques
In recent years, researchers in CT and MRI have tried many techniques to conclusively distinguish between benign and malignant cervical lymph nodes. These techniques represent the alternatives to PET/CT. PET has been shown to be superior to CT or MRI in the detection of recurrent tumors. Several studies have shown somewhat superior diagnostic accuracy for PET as compared to CT in the characterization of lymph nodes for the presence or absence of involvement with cancer.
Dynamic contrast techniques have been studied in both CT and MRI (11). These techniques rely on the more rapid contrast enhancement and more rapid contrast washout of malignant nodes. Repeat images are obtained at select cross-sectional levels over the course of a contrast bolus to determine the perfusion patterns of the tissues. These studies, when performed using the CT technique, do impart a higher radiation dose than simple static images.
Novel contrast agents, such as iron oxide particles, have been used in MRI (12). These agents interfere with the magnetic field when taken up by the normal lymphatic system. Lymph nodes replaced by tumor do not take up the contrast agent, and thus fail to drop signal. These techniques are promising, but the most promising contrast agent is not yet approved by the U.S. Food and Drug Administration.
MRI techniques such as magnetization transfer and T1-rho imaging showed initial promise in detecting metastatic nodes, but these techniques have fallen out of favor in recent years (13,14). Doppler sonography has been shown to detect and to some extent distinguish malignant nodes by their increased blood flow, but the inability to assess deep tissues and the strong operator dependence have limited this technique (15).
PET/CT in Evaluation of Patients with Head and Neck Malignancies
General
Studies have been performed over the past few years looking for potential added benefit with PET/CT over PET and CT performed separately. The use of combined PET/CT has reduced the incidence of pitfalls and allows easy differentiation of physiologic FDG uptake from pathologic uptake, more accurate localization of lesions, and detection of lesions that do not produce anatomic distortion (16,17,18,19,20,21,22,23). Physiologic structures such as muscle and brown fat are easily distinguishable from pathology on PET/CT, whereas with PET alone, with or without CT correlation, differentiation can be challenging or impossible and can lead to unnecessary further evaluation.
In terms of the overall added benefit of PET/CT compared to PET and CT interpreted separately in patients with head and neck cancer, a recent study from the University of Pittsburgh Medical Center showed the incremental value of PET/CT over PET and CT interpreted separately. PET/CT had an overall sensitivity of 98%, specificity of 92%, and accuracy of 94% for evaluating patients with known or suspected squamous cell carcinoma (SCC) of the head and neck (24). The study also showed that for all 125 lesions that were examined, CT was inferior to PET and PET was inferior to PET/CT and that 62% of lesions considered equivocal on CT and 41% of lesions equivocal on PET were categorized more definitively when radiologists had access to fused PET/CT images.
Another similar study by Schoder and Yeung (25) comparing PET and PET/CT in patients with any malignant lesion of the head and neck showed that PET/CT had a higher accuracy for depicting cancer than PET alone (96% vs. 90%). In this study 100 of 157 lesions had improved localization with PET/CT than with PET alone and 53% of equivocal lesions were classified more confidently based on PET/CT. These data demonstrate the superiority of PET/CT to PET alone and build on a large number of studies showing the superiority of PET to MRI or CT in several settings in patients with known or suspected head and neck cancers. These were extensively reviewed in the first edition of this book and only the more recent reports are emphasized in this update.
PET/CT in Unknown Primaries
Patients with head and neck SCC will often present with an enlarging neck mass. Fine-needle aspiration of the neck mass will reveal metastatic SCC, but because SCC does not arise de novo from lymph
nodes, the source of the disease may remain obscure. Usually clinical, radiographic, and endoscopic evaluation will reveal the primary tumor that is the source of the metastatic disease. In some cases, blind random biopsies of likely anatomic areas (tonsils, base of tongue) are used to find the primary tumor. Despite these efforts, unknown primary tumors account for up to 10% of head and neck SCC.
nodes, the source of the disease may remain obscure. Usually clinical, radiographic, and endoscopic evaluation will reveal the primary tumor that is the source of the metastatic disease. In some cases, blind random biopsies of likely anatomic areas (tonsils, base of tongue) are used to find the primary tumor. Despite these efforts, unknown primary tumors account for up to 10% of head and neck SCC.
There are several possible reasons for a primary tumor to remain unknown. Tumors less than 5 mm may be difficult to identify with endoscopy and cross-sectional imaging. Tumors embedded deep in the crypts of the palatine tonsils may escape notice. Clinicians accustomed to evaluating the head and neck can easily overlook cutaneous primary tumors and primary tumors outside the head and neck.
It is critically important for a primary tumor to be identified when metastatic SCC is discovered. The treatment of head and neck SCC focuses on surgical removal of the primary tumor and/or directed radiation therapy. If a primary tumor cannot be identified, the patient must undergo radiation of the entire upper aerodigestive mucosa. This results in considerable morbidity, particularly xerostomia and stenoses. More advanced treatment modalities, such as intensity-modulated radiation therapy (IMRT) and CyberKnife (Accuray, Sunnyvale, California) radiation, are not generally an option in patients with an unknown primary.
Many studies have addressed the efficacy of PET in evaluating patients with unknown primary tumors. These studies are difficult to compare because the definition of “unknown primary” varies from only clinical assessment to a full assessment with endoscopy and cross-sectional imaging. PET has been studied in this patient population and a sensitivity ranging from 5% to 60% was found, but larger studies suggested a sensitivity in the range of 25% to 35% (26,27,28,29,30,31,32,33). Preliminary studies suggest that PET/CT may offer a slight increase in overall sensitivity (33% to57%) for detecting unknown primary tumors, but may offer significantly more information regarding biopsy localization information (Fig. 8.7.8) (34,35).
The major pitfall for identifying the primary tumor in the setting of known SCC metastases is the variable physiologic uptake of FDG in the common locations of hidden primary tumors. In particular, the palatine tonsils and the lingual tonsil at the tongue base can have variable (and asymmetric) uptake in normal individuals, and this uptake may mask an underlying lesion. In this situation, PET/CT has an advantage over PET because the presence or absence of a mass on the CT can lend credence to asymmetric FDG uptake. Alternatively, physiologic uptake may be mistaken for tumor.
The role of PET/CT in the assessment of the unknown primary is to lead the surgeon to potential sources of tumor. As such, these examinations should be read with high sensitivity, because the risk of a negative biopsy is worth the potential benefits of identifying the primary lesion.
Role of PET/CT in Staging
Many patients who are candidates for staging PET/CT will have already undergone a CT of the neck. Patients who are seen at primary care hospitals often begin their radiographic work-up at those sites. Also, if a patient’s diagnosis is in question, a CT may be performed before it is clear that a PET/CT would have been the better choice.
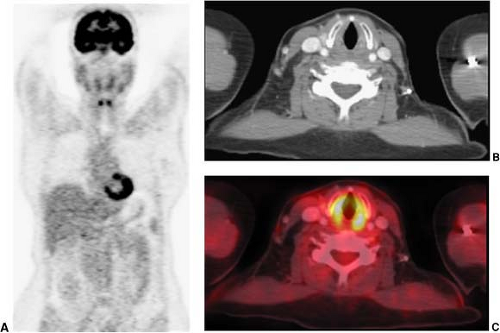
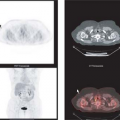
Not all patients who have already undergone a good quality neck CT require an additional staging PET/CT with a contrast-enhanced CT. Because traditional cross-sectional imaging is usually sufficient to evaluate the primary lesion, typically only patients who are at substantial risk of nodal or hematogenous metastases would benefit from PET/CT. In this patient population, it is not uncommon to find metastases outside of the head and neck (Fig. 8.7.9). Since many of the risk factors for head and neck cancer are also risk factors for lung cancer, the detection of additional primary cancers is also not uncommon when PET/CT is used for tumor staging.
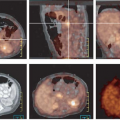
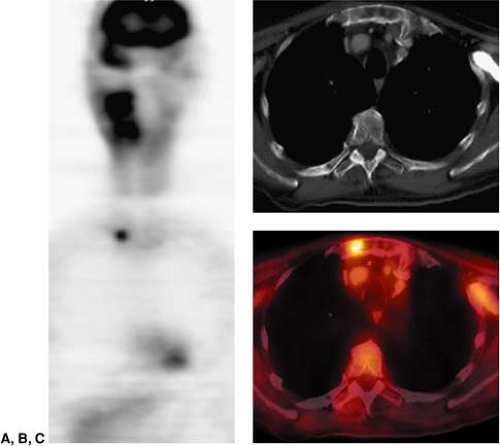 Figure 8.7.9. Detection of lesions outside of the neck. Coronal PET image (A) demonstrates a focal area of intense fluorodeoxyglucose (FDG) activity in the thorax, but the precise localization cannot be determined. This patient was referred for restaging PET/CT of squamous cell carcinoma of the right oropharynx. Axial CT (B) shows no definite abnormalities in the thorax. The fused PET/CT image (C) shows a focal area of intense FDG activity in the sternum just to the left of the right sternoclavicular joint, compatible with a metastatic lesion outside of the neck. (From Fukui MB, Blodgett TM, Snyderman CH, et al. Combined PET/CT in the head and neck: part 2. Diagnostic uses and pitfalls of oncologic imaging. RadioGraphics 2005;25:913-930, with permission.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|