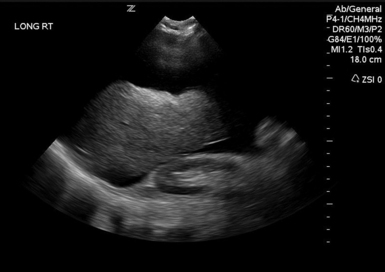
46 Intraabdominal hypertension (IAH) and abdominal compartment syndrome (ACS) are well-documented causes of morbidity and mortality in the critically ill. IAH and ACS may affect almost every organ system.1 The World Society of the Abdominal Compartment Syndrome (www.wsacs.org) has set forth a number of definitions based on clinical evidence and expert opinion. Intraabdominal pressure (IAP) is normally 5 to 7 mm Hg in adults. IAH is defined as sustained or repeated pathologic evaluation of IAP greater than or equal to 12 mm Hg. This is further subdivided into four grades based on pressure value. ACS is sustained IAP greater than 20 mm Hg, associated with new organ dysfunction/failure.1 Usual causes of IAH and ACS include intraabdominal hemorrhage, pneumoperitoneum from perforated viscus, and third spacing of fluid during massive resuscitation (Figure 46-1). Cardiac dysfunction occurs in patients with IAH and ACS because of increased intrathoracic pressure (ITP) resulting from upward diaphragm displacement. This increased ITP causes a significant decrease in venous return, thereby reducing cardiac output. There is a clear association between IAP and ITP. Fifty percent of the IAP is transmitted and affects the ITP.2 Cheatham et al3 point out that catheter-based hemodynamic measures, such as pulmonary artery occlusion pressure and central venous pressure, therefore have significant limitations as indexes of volume status in the face of IAH. They proposed the idea of using volumetric measurements, such as end-diastolic volume index, in directing adequate resuscitation in these patients. Anticipating the relationship between IAP and ITP and possibly using volume indexes for estimation of preload could help to prevent inadequate resuscitation and inappropriate use of vasoactive agents in patients with IAH. Several ultrasound (US) methods are used to guide therapeutic strategies in the face of IAH. Resuscitation efforts geared toward a right ventricular, end-diastolic volume index, goal-directed model have been shown to reduce the incidence of multiple organ failure and death.4 IAH results in increased ITP by pulmonary parenchymal compression and reduced chest wall compliance. Parenchymal compression leads to pulmonary hypertension. To overcome the alveolar compression and atelectasis, positive end-expiratory pressure (PEEP) is increased or added to maintain oxygenation and ventilation. Aggressive PEEP can result in not only opening up atelectatic areas of the lung but also overdistending normal lung and inhibiting adequate ventilation.1,5 An early sign of end-organ hypoperfusion resulting from IAH and/or ACS is decreased urine output. Renal dysfunction usually occurs with oliguria at 15 mm Hg and anuria at 30 mm Hg in euvolemic patients with no underlying kidney disease.6 This may occur at lower IAP in those who are already hypovolemic or in those with baseline pathology. Dysfunction may be due to compression of the renal parenchyma and vein, along with decreased renal perfusion pressures, leading thus to microcirculatory alterations and decreased urine output.7 Urine output is dependent on the renal filtration gradient (FG) or the glomerular filtration pressure (GFP) minus the proximal tubular pressure (PTP), which can be estimated as mean arterial pressure (MAP) minus two times the IAP [FG = GFP − PTP = MAP − (2 × IAP)].1,6 Measurements are performed at the end of expiration in a supine position with the transducer zeroed at the iliac crest in the midaxillary line by using 25 mL of saline instilled into the bladder. The measurement is taken in mm Hg between 30 to 60 seconds after the saline is instilled and catheter clamped. All abdominal muscle contractions should be absent during measurements.8,9 Intragastric pressure (IGP) monitoring has been used, in place of bladder pressure monitoring, in several experimental models to obtain abdominal pressure readings.10,11 There is a close relationship between IAP and IGP, with potential for inaccuracy in patients with ileus and in those who are being enterally fed.11 Recently, abdominal US has been used to identify patients with IAH and ACS. Cavaliere’s group12 examined the ultrasonographic detection of changes in the dimensions of abdominal veins—in the setting of simulated increased IAP in normal volunteers—as a marker of ACS. They used a pelvic binder to create external compression, inducing mild IAH. Dimensions of multiple abdominal veins were estimated by B-mode, while pulsed wave Doppler measured peak blood flow velocities at the end of expiration. The inferior vena cava (IVC) below the renal veins, the right suprahepatic vein, the portal vein (PV), the right external iliac vein, and the segmental branches of the right renal artery were measured. Significant changes in IVC and PV diameters were noted. Diameters were noted to be decreased about 10%, whereas the cross-sectional area of the IVC was decreased about 25%. No significant changes were evident in peak Doppler velocities of the studied vessels, but significant variability between individuals existed. Studies using computed tomography scans revealed distorted IVC shape and decreased diameter as well as flattening of renal vasculature in ACS.13 Further research should include ultrasonographic analysis of IVC and PV in patients with IAH and ACS.
Use of ultrasound in the evaluation and treatment of intraabdominal hypertension and abdominal compartment syndrome
Overview
Pathophysiologic effects
Cardiovascular
Respiratory
Renal
Ultrasound and other diagnostic adjuncts
Intravesicular pressure
Intragastric pressure
Abdominal ultrasonography
Use of ultrasound in the evaluation and treatment of intraabdominal hypertension and abdominal compartment syndrome