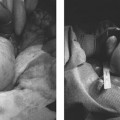
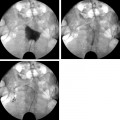
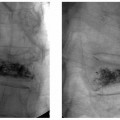
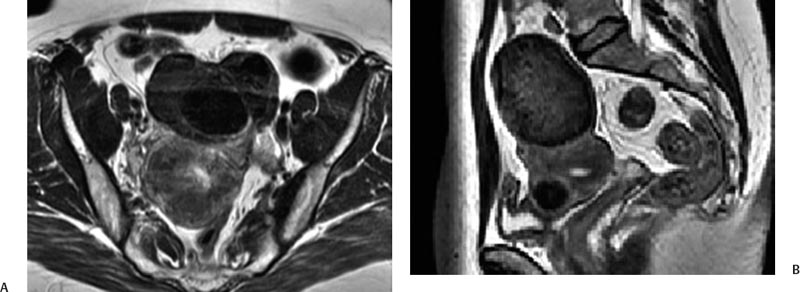
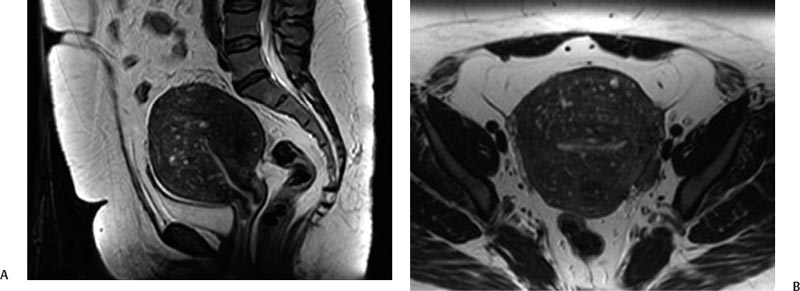
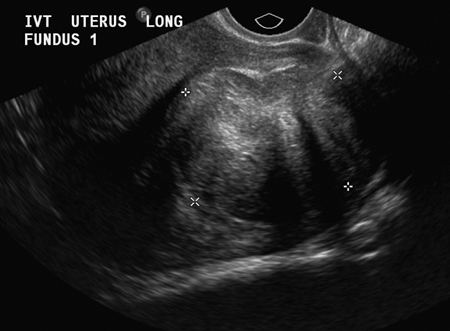
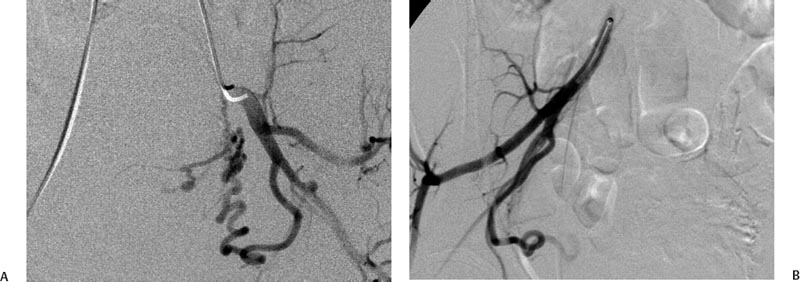
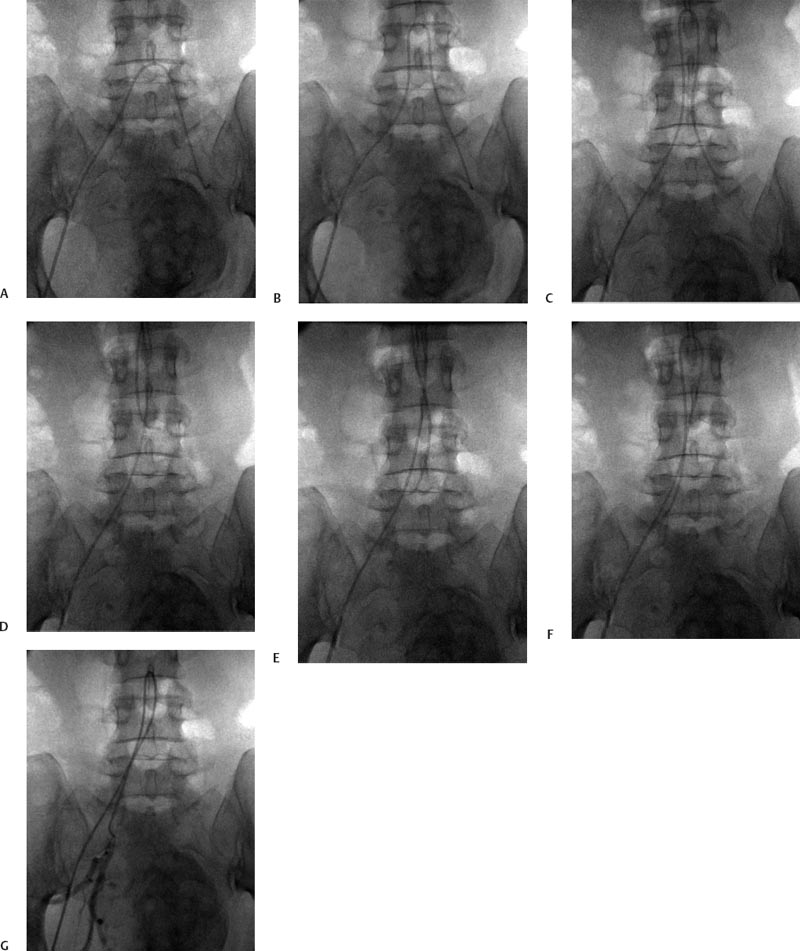
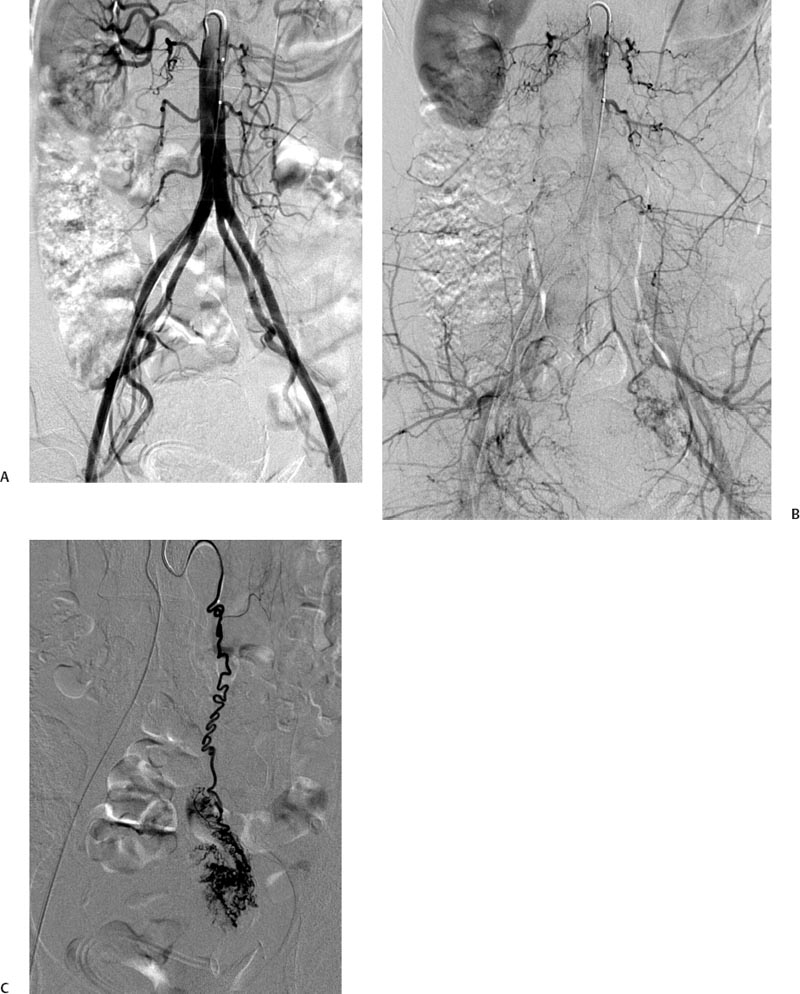
5 Uterine Fibroid Embolization Gary P. Siskin Ravina et al and Goodwin et al have been credited with introducing uterine fibroid embolization (UFE) to interventional radiology (IR).1–3 Since their respective publications in the mid-1990s, UFE has enjoyed growing acceptance as an alternative to the surgical resection of symptomatic uterine fibroids.4,5 In fact, Jacobson et al6 recently demonstrated the growing role that UFE is playing as a uterine-conserving option for these patients. In their review of patients in northern California, they found that though the total rate of invasive treatment for fibroids has stayed constant in recent years, the rate of hysterectomies decreased from 2.13 per 1000 patients to 1.91 per 1000 patients. At the same time, the rate of UFE procedures increased from less than 0.1 per 1000 patients to 0.24 per 1000 patients. Hence, UFE alone is responsible for decreasing the hysterectomy rate in this population; the effect is likely similar wherever UFE is being offered. The UFE procedure is indicated for the treatment of symptomatic uterine fibroids. The symptoms that are associated with uterine fibroids that might prompt a patient to discuss treatment options with her physician include abnormal uterine bleeding, pelvic pain, dyspareunia, abdominal distension, and frequent urination. Infertility and pregnancy-related concerns may also lead to discussions regarding treatment options for uterine fibroids. It is important to address the issue of performing this procedure in patients without symptoms as simply a way to address the “presence” of a fibroid within the uterus. This has not yet been established as an indication for UFE; therefore, although exceptions can be made on a case-by-case basis, UFE is probably best utilized as a treatment for patients with symptomatic fibroids as opposed to asymptomatic fibroids. As an angiographic procedure, some possible relative contraindications must be considered prior to performing a UFE procedure. Because iodinated contrast is utilized during this procedure, a patient’s renal status must be considered prior to the procedure. Although most of the patients undergoing UFE are young and healthy individuals, some patients may have or be at risk for renal insufficiency and an evaluation of renal function is recommended in these individuals prior to the use of iodinated contrast. Similarly, patients felt to be at risk for bleeding complications relating to performance of an arterial puncture into the common femoral artery should have their hematologic status evaluated prior to performance of the procedure. Finally, patients with an allergy to iodinated contrast material will likely have difficulty undergoing this angiographic procedure; however, performing this procedure without the use of iodinated contrast material has been described.7,8 There are uterine or fibroid issues that may serve as relative contraindications for the performance of a UFE procedure. Some patients will present with pedunculated fibroids, which are connected to the uterus via a stalk (Fig. 5.1). Pedunculated subserosal fibroids have generally been recognized as a relative contraindication for UFE.9 It is felt by some that embolization may lead to necrosis and disruption of the stalk, causing the fibroid to separate from the uterus and be free within the peritoneal cavity. There are cases that have been reported where hysterectomy and bowel resection have been performed after septic necrosis of pedunculated subserosal fibroids.10,11 Therefore, recent recommendations have been made to assess the diameter of the stalk relative to the diameter of the fibroid before performing a UFE procedure on a patient with a pedunculated fibroid. Katsumori et al12 reported the safety of performing UFE in patients with pedunculated subserosal fibroids that have a stalk diameter of 2 or more centimeters. In their series, they encountered no serious complications, such as separation of septic necrosis of pedunculated fibroids, torsion of the tumor, infection, or increased tumor size caused by liquefied change as reported by Walker et al.13 Margau et al14 reported no unique complications after UFE of pedunculated subserosal fibroids with stalk diameters ranging from 0.7–7.8 cm. Others have discussed the safety of performing UFE if the diameter of the stalk is greater than one-third the width of the diameter of the fibroid. If the diameter of the stalk is less than one-third the width of the diameter of the fibroid then consideration should be given toward recommending laparoscopic resection of the fibroid. In the case of pedunculated submucosal fibroids, Verma et al15 evaluated the relationship between the fibroid and endometrium and determined the ratio between the largest endometrial interface and the maximum dimension of the fibroid. A stalk diameter >45% was found to decrease the risk of endocavitary migration after UFE. Thinner stalks should necessitate consideration of a hysteroscopic myomectomy. Fig. 5.1 (A) Axial and (B) sagittal T2-weighted magnetic resonance images of the pelvis demonstrating pedunculated subserosal fibroids with a broad-based attachment to the uterus (>50% of the diameter of the fibroid). The presence of adenomyosis within the uterus is considered controversial within the IR community as to whether or not this actually is a contraindication to UFE. Adenomyosis is a disorder that is characterized by the presence of endometrial islets within the myometrium. By definition, they pathologically consist of epithelial as well as stromal elements and are situated at least 2.5 mm below the endometrial–myometrial junction.16 Clinically, adenomyosis can result in abnormal bleeding, pain, and bulk-related symptoms that may be similar to those seen in patients with uterine fibroids. On magnetic resonance imaging (MRI), T2-weighted images are useful in identifying a junctional zone with a thickness >12 mm and oftentimes containing subendometrial cysts (Fig. 5.2). Fig. 5.2 (A) Sagittal and (B) axial T2-weighted magnetic resonance images of the pelvis demonstrating a thickened junctional zone containing sub-endometrial cysts seen as punctuate foci of high signal. These findings are consistent with diffuse adenomyosis. Initially, cases of treatment failure after UFE were attributed to the presence of adenomyosis; therefore, adenomyosis was felt to be a contraindication to this procedure.17,18 Later studies, in the form of small retrospective case series, demonstrated the potential for patients with adenomyosis, with or without the presence of uterine fibroids, to benefit from embolization.19–21 Additional data has been published over the course of the last several years, which continue to support the use of embolization in this population of patients, albeit with expectations regarding success that are less than that seen with embolization in patients with fibroids without adenomyosis.22–25 This is especially true when adenomyosis exists alone within the uterus, without the presence of uterine fibroids. For example, Lohle et al23 studied 38 patients with symptomatic adenomyosis with or without fibroids. After a median follow-up of 16.5 months, 84.2% of patients were satisfied with the clinical improvement they experienced after embolization. On the other hand, Pelage et al25 published a prospective study of 18 patients with adenomyosis and no fibroids. In this study, embolization resulted in good short-term control of symptoms with 94% of patients reporting improvement or resolution at 6 months. This number, however, decreased with time and only 55% were clinically improved after 2 years of follow-up. Similarly, Kim et al24 studied 54 patients with adenomyosis and no fibroids who underwent UAE and had a minimum of 3 years of follow-up. In this patient population, 57.4% of patients had long-term success documented as improvement in menorrhagia and dysmenorrhea scores. The remaining patients had a mean of 17.3 months between embolization and symptom recurrence. These results have been viewed as suboptimal in the IR community, but interestingly are viewed positively within the gynecology community as possible evidence that embolization can be considered as a nonsurgical option for this difficult patient population.26 Therefore, with such discrepant interpretations offered for these results it seems fair to say that UAE can be considered as treatment for patients with symptomatic fibroids and adenomyosis. The results seem only slightly inferior to those seen in patients with fibroids and no evidence for adenomyosis. In addition, results in patients with adenomyosis and no evidence for uterine fibroids seem to leave approximately one-half of patients with recurrent symptoms over time. Therefore, the decision to perform embolization should be made on a case-by-case basis because a success rate of 50% of patients may be appropriate in some patients and not appropriate in others. The presence of extremely large fibroids has been considered by some to be a contraindication to the performance of UFE. The literature supporting this has been mixed. Prollius et al27 reported on the outcomes of UFE in patients with a uterus greater than the size of a 24-week gestation (volume >780 cm3) and compared them to patients with smaller uteri. There was no difference in the complication rate, in the degree of symptomatic improvement, and satisfaction after the procedure between the two groups of patients. However, Spies et al, in their study describing long-term outcomes after UFE, demonstrated that patients with very large uteri and large dominant leiomyomata are among those that are more likely to have recurrence and subsequent intervention.28 Therefore, patients with large uteri and dominant fibroids need to be counseled regarding the uncertainty of their clinical outcome after UFE. Additional contraindications include the presence of a viable intrauterine pregnancy for obvious reasons. An active, untreated infection represents a contraindication due to the risk of abscess formation and related septic complications.9 Similarly, chronic endometritis, hydrosalpinx, or partially treated pelvic infections represent contraindications as well.29 The question of whether or not UFE is appropriate to perform in a patient with a desire to have children in the future is one that frequently arises in the course of today’s IR practice and is addressed in Chapter 7. Prior to the performance of a UFE procedure, patients should be seen and evaluated in an outpatient setting, preferably in an office-based setting that is conducive to performing patient assessments and maintaining continuity of care.9,30 The purpose of this visit is to determine if the embolization procedure is indicated and to determine if it is the best and most appropriate option for the patient. During this visit, the patient’s history is reviewed (including the results of her most recent gynecologic examination, which should be within the last 12 months) and a physical examination is performed. All prior tests, including the results of a recent Papanicolaou (Pap) test, endometrial biopsy, and blood test results should be reviewed. The patient should have a normal Pap test result within 12 months before embolization is performed.9 Patients who present with a history of continuous bleeding, very prolonged menstrual periods, significant intermenstrual bleeding, or bleeding after menopause should undergo an endometrial biopsy prior to UFE due to their risk for endometrial hyperplasia or endometrial malignancy; postmenopausal patients with bulk-related symptoms have been shown to benefit from UFE.31 Old imaging studies should be reviewed as well. From the perspective of a radiologist, films rather than reports should be reviewed when it comes to previously performed imaging studies. Only after that is completed can a physician provide the patient with an appropriate recommendation regarding treatment.9 For most patients, routine blood work is not necessarily indicated. Certainly if a patient is at risk given their medical history for bleeding issues associated with a femoral puncture or renal failure secondary to the use of iodinated contrast, then blood work should be obtained to assess their coagulation status (platelet count, activated partial thromboplastin time, prothrombin time, and international normalized ratio) or baseline renal function (blood urea nitrogen and creatinine levels). It is helpful for comparative purposes to obtain a complete blood count (CBC) in patients with a history of abnormal bleeding. Some of the most interesting investigative work concerning UFE has been surrounding the role of imaging in the pre- and postprocedure assessment of patients undergoing this procedure. There is certainly consensus that preprocedure imaging is required to both confirm the diagnosis of uterine fibroids and to exclude the presence of coexisting pathology. When hysterectomies were routinely being performed for patients requiring treatment for fibroids, great imaging was not necessarily needed prior to surgery. The uterus and possibly the ovaries were going to be removed, and the rest of the pelvis was going to be explored. It was therefore going to be the responsibility of a pathologist to determine what problems existed within the uterus that caused the patient’s presenting symptoms. With an organ-sparing philosophy now more prevalent, especially when it comes to UFE, the interventional radiologist performing this procedure has the obligation to obtain the best possible images to make the final determination as to whether or not UFE is the most appropriate treatment to address a patient’s presenting symptoms. The question that then comes up is which modality best serves this purpose and can best answer these questions. In most gynecology practices, the standard of care is to utilize ultrasound to evaluate patients with symptomatic uterine fibroids. This modality has been shown to effectively demonstrate the presence of fibroids and can be utilized to determine their size and their location within the uterus (Fig. 5.3). Ultrasound can also be used to characterize the endometrium and the ovaries (which may be difficult in the presence of uterine fibroids). It has also been established that ultrasound is operator dependent, may be limited by the patient’s body habitus, may not be able to depict coexistent pelvic disease (such as endometriosis), and may be less sensitive at diagnosing adenomyosis.31–34 Spielmann et al35 retrospectively reviewed the imaging studies of 49 women who were referred for consultation for UFE. They found that MRI discovered additional fibroids in 31 of 49 patients when compared with ultrasound. In addition, they found that fibroids were diagnosed in error on ultrasound in five patients; no fibroids were found on MRI in these patients. Importantly, they found that MRI significantly affected the evaluation of fibroid size and location within the uterus as well. Therefore, this further supports the fact that ultrasound may not be able to supply all of the information an interventional radiologist needs to determine if a patient is an appropriate candidate for UFE. Fig. 5.3 Longitudinal image of the uterus from a transvaginal pelvic ultrasound demonstrating an intramural intrauterine mass consistent with a uterine fibroid. Magnetic resonance imaging has been shown to be the most effective modality for evaluating a patient prior to UFE. Because of soft tissue characterization, multiplanar imaging capabilities, and enhancement, MRI not only accurately detects and characterizes uterine fibroids, but also may predict who will benefit from embolization.36 In addition, the ability of MRI to detect coexistent uterine or pelvic pathology may change the diagnosis and treatment plans for patients being evaluated for UFE. The use of MR angiography may be useful in assessing pelvic vascular anatomy before the procedure and in identifying collateral vessels to the uterus.37 Omary et al38 effectively studied the effect of preprocedural MRI on the diagnosis and treatment of patients being evaluated for UFE. In this study, MRI changed the initial diagnosis in 18% of patients. A significant number of patients (19%) who were initially referred for UFE were felt to no longer be appropriate candidates for this procedure after the MRI was performed for reasons such as large fibroids, adenomyosis, pedunculated submucosal fibroids, endometrial lesions, etc. Instead, these patients were referred for surgery, clinical management, or biopsy rather than UFE. Nikolaidis et al39 expanded upon the previous study by assessing the incidence of nonviable fibroids in patients referred for UFE. In their study, they found that 6% of patients had nonviable dominant fibroids based on a lack of enhancement on postcontrast images. Given the fact that vascularity is intuitively necessary for embolization to be effective, fibroids without significant vascularity will likely not respond to embolization. Therefore, this information was valuable to these investigators because the decision was then made to not offer UFE to these patients. This once again demonstrates that the information obtained on an MRI has a significant likelihood of altering the treatment plan for a patient being considered for UFE. There have been some reports at using the findings on a preprocedure MRI to predict outcome after UFE. Harman et al40 found that the signal characteristics and contrast-enhancement patterns of fibroids before embolization can predict the degree of tumor volume after UFE. Volume reduction was more prominent in fibroids that had high signal intensity on T2-weighted images and a marked contrast enhancement on T1-weighted images. However, the volume reduction was insufficient in fibroids with high signal characteristics on precontrast T1-weighted images. Burn et al41 found that before embolization, high signal intensity on T1-weighted images was predictive of a poor response, whereas high signal intensity on T2-weighted images was predictive of a good response. The degree of gadolinium enhancement did not correlate with fibroid volume reduction. Jha et al42 also identified pretreatment MRI features that may be predictive of successful UFE. In this study, a submucosal location was a strong positive predictor of fibroid volume reduction after UFE. Prior to beginning the procedure, patients are reevaluated in a preprocedure holding area. Informed consent is obtained. An intravenous line is placed to enable the patient to be hydrated and to receive medication for conscious sedation during the procedure (such as midazolam and fentanyl in most centers). In most centers, prophylactic antibiotics are given, but this is not universal among centers performing UFE.43 Although evidence from randomized controlled trials is lacking, most centers choosing to use prophylactic antibiotics select cefazolin, based on the fact that the most likely source of pathogens during solid organ embolization is contamination by skin pathogens (Staphylococcus or Streptococcus).43 As an angiographic procedure, arterial access is typically gained via the right common femoral artery and unilateral access is maintained throughout the entire procedure. Spies et al has described use of bilateral common femoral artery access with two operators to reduce fluoroscopic exposure (by performing the right and left UAE at the same time) and to allow for bilateral contralateral catheterizations of the uterine artery instead of one contralateral and one ipsilateral catheterization.28 The embolization procedure itself is performed using standard angiographic technique. Seldinger technique is used to gain access into the right common femoral artery (assuming unilateral access). A 5-French sheath is used to maintain access within the common femoral artery. At my institution, a 5-French Cobra Glide (Terumo Medical Corporation, Somerset, NJ) catheter is used to catheterize the left internal iliac artery. A pelvic arteriogram is performed to evaluate the left-sided pelvic arterial anatomy and to localize the origin and course of the left uterine artery (Fig. 5.4A). Under road-mapping guidance, a microcatheter with a 0.027-inch inner luminal diameter is then introduced into the 5-French Cobra catheter using coaxial technique. This catheter, together with a 0.018-inch guide-wire, is advanced into the left uterine artery, which classically arises as the first or second branch of the anterior division of the internal iliac artery.44 If the cervicovaginal branch of the uterine artery, which typically arises from the mid to distal portion of the transverse segment of the uterine artery, is visualized, then the tip of the microcatheter is positioned beyond the origin of that artery.45 This is done to reduce the possible occurrence of sexual dysfunction following UFE, a complication which has been attributed to embolization of this branch of the uterine artery.46 Fig. 5.4 (A) 30-degree left anterior oblique and (B) right anterior oblique images from selective left and right internal iliac artery angiograms demonstrating the origin of the uterine artery from the anterior division of the internal iliac artery. Fig. 5.5 (A-G) Multiple fluoroscopic images demonstrating the use of the Waltman Loop technique to move an angiographic catheter from the left internal iliac artery to the right internal iliac artery prior to selective catheterization of the right uterine artery. Once the microcatheter is in an appropriate position, the embolic agent selected for use is administered into the uterine artery until the endpoint signifying an appropriate reduction in blood flow has been reached. The most common endpoint to use with particulate polyvinyl alcohol (PVA) is complete stasis of flow within the uterine artery. If a spherical embolic agent, such as tris-acryl gelatin microspheres, is used, then embolization is stopped when there is occlusion of the uterine arterial branches with slow antegrade flow in the main uterine artery.43 This slow flow can be characterized by visualizing contrast within the main uterine artery for 5 to 10 heartbeats following injection into the uterine artery. At this point, the micro-catheter is removed and the Cobra catheter is repositioned into the right internal iliac artery, using the Waltman Loop technique (Fig. 5.5).47 The right uterine artery is then selectively catheterized with the microcatheter and embolization of the right uterine artery is performed (Fig. 5.4B). The question as to whether or not both uterine arteries need to be embolized is a fair one to ask. Even in Goodwin’s initial article on UFE in the United States, the utility of bilateral embolization was discussed.3 The one patient in this limited series that failed after UFE underwent only a unilateral embolization. Recently, Nicholson48 reported mixed results when unilateral embolization is performed due to technical reasons. In addition, Gabriel-Cox et al49 reported that unilateral uterine artery embolization predicted subsequent hysterectomy for patients undergoing UFE. Other factors such as age, indication for UFE, uterine volume, embolic agent utilized, and radiologist experience did not predict subsequent hysterectomy for this patient population. This once again demonstrated the importance of a bilateral uterine artery embolization during UFE. Once both uterine arteries have been embolized, it is fairly typical for a completion abdominal aortogram to be performed. This aortogram can be used to confirm that flow is slowed or stagnant within both uterine arteries. It is also helpful to potentially determine if significant collateral vessels are present to the uterus and the fibroids.50,51 Specifically, it can help determine if dilated ovarian arteries are present, which are contributing to the arterial supply of the fibroids, especially once the uterine arteries have been embolized (Fig. 5.6). The utility of a completion aortogram is not universally agreed upon. White et al52 retrospectively reviewed 1072 UFE patients to identify patients in whom ovarian arteries were identified and contributed significantly to pelvic arterial flow. They found that on aortography, only 0.8% of patients had ovarian arteries identified, which supplied arterial flow to >10% of the uterus. When the ovarian arteries identified were selectively catheterized and angiograms were performed, 5.8% of patients were felt to have significant ovarian artery collateral supply to the uterus. Therefore, the sensitivity of aortography was only 18%. This fact, together with the findings that aortography contributes a substantial amount of radiation (>20% of total) to the overall exposure experienced during UFE,53 has led some to believe that the routine performance of postembolization aortography may be of limited utility and should possibly be reconsidered. In fact, some are now advocating that magnetic resonance angiography (MRA) be used prior to UFE procedures to evaluate for the possibility of significant ovarian arterial collateral flow to fibroids.54 The determination as to whether or not a prominent ovarian artery should be embolized is one that may have to be made in response to the findings on an abdominal aortogram or selective ovarian arteriogram. Abbara et al55 recommended that selective ovarian arteriography be performed if large ovarian arteries with rapid flow extending into the pelvis are identified on aortography. This allows one to determine if the ovarian arteries are responsible for some of the arterial supply of the treated fibroid. It is known that ovarian artery supply can result in treatment failure after UFE, which is why ovarian artery embolization has been used with success in these patients.55,56 A classification system developed by Razavi et al57 can potentially help with deciding if an ovarian artery needs to be embolized. In this article, three types of anastomoses were identified. In type I anastomoses, flow from the ovarian artery to the uterus was through anastomoses with the main uterine artery. In type II anastomoses, the ovarian artery supplied the fibroids directly. In type III anastomoses, the major blood supply to the ovary was from the uterine artery. Given this system, identification of a type II anastomoses would likely warrant ovarian artery embolization to be certain that the entire blood supply to the fibroid(s) is addressed. Particulate PVA was the agent used historically and was the agent that when used, provided the success of UFE as a treatment option for this patient population. Its ability to address the symptoms of a patient with uterine fibroids and decrease uterine and fibroid volume has been well documented.3,58–62 As a result, during the early years of UFE, there was virtual agreement that particulate PVA was the most appropriate agent to use for this procedure. In addition, the endpoint of stasis of flow within the uterine artery was agreed upon because it was the endpoint used for most embolization procedures at the time and was the most reliable endpoint to achieve with the use of particulate PVA. However, there are known shortcomings to particulate PVA that prompted investigation into optimizing the agent used during this, and other embolization procedures. These shortcomings include the inherent size variability in particle preparations, the known difficulty of injecting particulate PVA through a microcatheter, and the clumping of particles that makes the effective size of PVA larger than the actual size, leading to an embolic occlusion that is more proximal than intended.63–66 Fig. 5.6 (A,B) Two views from an abdominal aortogram after bilateral uterine artery embolization demonstrating significant flow in the left ovarian artery that is associated with an angiographic blush within the uterus. (C) A selective angiogram of the left ovarian artery confirming the role that this vessel plays in supplying arterial blood to the fibroid. This vessel was embolized using 500 to 700 micron Embosphere Microspheres (Biosphere Medical Corp, Rockland, MA) after this angiogram. Once trisacryl gelatin microspheres (Embosphere Microspheres, Biosphere Medical Corp., Rockland, MA) were introduced as an alternative embolic agent to particulate PVA, embolic agent selection has been a point of controversy among interventional radiologists. This agent has been associated with significant clinical success when used for UFE (Fig. 5.7).67–71 The spherical configuration of this agent was perceived as being advantageous because it addressed many of the above-listed shortcomings of particulate PVA. For example, Chua et al72 reported that the tris-acryl gelatin microspheres measuring 700 to 900 microns in diameter penetrated deeper into the circulation of fibroids when compared with particulate PVA measuring 355 to 500 microns in diameter, implying less proximal aggregation associated with the gelatin-based micro-spheres. Although Spies et al failed to show any substantive differences in outcomes between the use of tris-acryl gelatin microspheres and particulate PVA, Smeets et al did demonstrate a slightly increased improvement in patient satisfaction when the gelatin-based microspheres were used.73,74 The clinical success of trisacryl gelatin microspheres prompted others to develop spherical agents for this procedure. PVA-based microspheres (including Contour SE Microspheres, Boston Scientific Corp., Natick, MA, and Bead Block, Terumo Corp., Somerset, NJ), were felt to have potential for this procedure because of their spherical configuration and the comfort and familiarity that interventional radiologists have had through the years with PVA. As experience with these new PVA-based microspheres increased, sentiment grew within the IR community that these products were not appropriate for UAE.75,76 This was based on decreased clinical efficacy of these products in addition to a failure to achieve fibroid infarction to the same degree that is seen with trisacryl gelatin micro-spheres.77–82 Therefore, at the present time, trisacryl gelatin microspheres are considered to be the embolic agent of choice for UFE given its success at achieving improvement in presenting symptoms, reduction of uterine and dominant fibroid volume, and infarction of the fibroids within the uterus at the time of treatment. Fig. 5.7 One syringe of 500 to 700 micron trisacryl gelatin microspheres (Embosphere Microspheres, Biosphere Medical Corp., Rockland, MA). One consistent feature of UFE, and one that is often the focus of patients considering this procedure and physicians discussing this procedure, is the experience of patients during the immediate postprocedure recovery period. Through the years, it has been well established that solid-organ embolization is associated with a constellation of symptoms known as the postembolization syndrome. Uterine fibroid embolization is no exception. Following UFE, patients typically experience symptoms including pelvic pain or cramping, nausea and vomiting, low-grade fever, fatigue, and generalized malaise. These symptoms last a variable amount of time across different patients, but one can generally expect the symptoms to last anywhere from 3 to 7 days. In most patients, pain generally increases over the first 2 hours after the procedure is completed and then plateaus for several hours. It then decreases fairly rapidly to a much lower level, typically following the first 8 to 10 hours after the procedure.83 It has been shown that the severity of pain experienced after UFE cannot be predicted based on baseline uterine or fibroid volume and that the severity of pain experienced cannot be used to predict the clinical outcome from this procedure.84 Spies et al and Ryu et al have shown that the amount of pain experienced after UFE does not appear to be different when particulate PVA or trisacryl gelatin microspheres are used for the procedure.85,86 However, Hovsepian et al87 demonstrated that patients embolized with trisacryl gelatin mi-crospheres experienced more pain than those embolized with PVA-based microspheres. Volkers et al,88 reporting data from the EMMY trial, revealed that there was a relationship between the amount of embolic agent used and the risk for severe pain, postprocedural fever, and major complications.
Indications and Contraindications
Preprocedure Patient Evaluation
Technique
Embolic Agent Selection

Postprocedure Recovery
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree