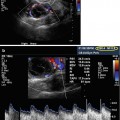
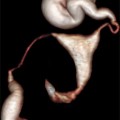
Fig. 10.1
Saline infusion sonohysterography with intramural fibroid
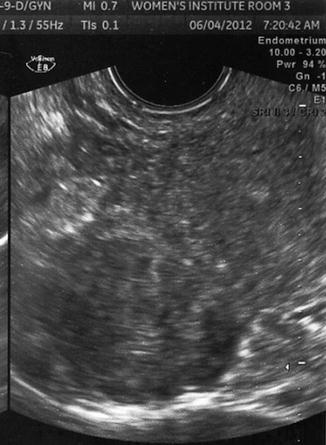
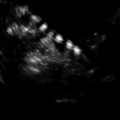
Fig. 10.2
Transvaginal ultrasound demonstrating subserosal fibroid
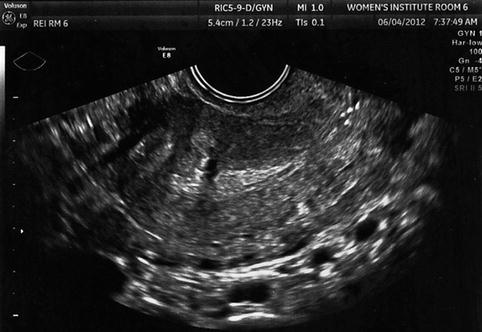
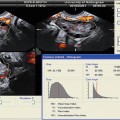
Fig. 10.3
Transvaginal ultrasound demonstrating several uterine fibroids, including one submucous myoma with deflection of the endometrial cavity
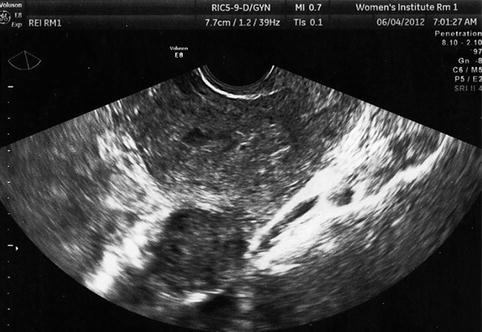
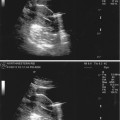
Fig. 10.4
Transvaginal ultrasound demonstrating pedunculated fibroid
Symptoms attributed to fibroids are determined by the size and the location of the masses. Most intramural, subserosal, and pedunculated fibroids are asymptomatic. However, large fibroids may cause bulk symptoms such as abdominal pressure, bloating, or distention. A myoma that presses against the bladder may cause urinary frequency, urgency, or nocturia. A fibroid that compresses the rectum may cause constipation, diarrhea, or alternating symptoms. Infarction of a fibroid may cause severe acute pain, and the inflammation caused by degenerating myoma may cause adhesions. Fibroids located in the posterior cul de sac may cause dyspareunia. Occasionally, fibroids are associated with chronic, intermittent, or cyclic pain.
Submucous myomas often cause abnormal uterine bleeding. Symptoms of submucous fibroids include menorrhagia, dysmenorrhea, clotting, and intermenstrual bleeding [8]. When bleeding is severe, anemia may occur. With the high prevalence of fibroids and the multitude of symptoms that may be attributed to fibroids, it is not surprising that fibroids are the leading indication for hysterectomy. However, other treatment options must be considered for women who are interested in childbearing.
Fibroids and Fertility
It is difficult to determine the direct impact of fibroids on fertility, since the incidence of uterine fibroids increases with age, fertility declines with age, and many women with fibroids conceive spontaneously.
The location of fibroids is important in determining the impact on fertility. In some circumstances, fibroids impair fertility by mechanically distorting the uterine cavity, altering the endometrium, and impairing embryo implantation and growth. Other obvious causes of fibroid-related infertility may include mechanical obstruction of the tubal ostia.
Submucous myomas directly impair fertility and cause adverse reproductive outcomes by several potential mechanisms [9]. These fibroids alter the vascular supply and development of the endometrium with intramural myomas or alter growth factors and inflammatory substances that may impair implantation or fetal growth. The mechanical distortion of the endometrial cavity almost certainly has a direct effect on fertility. In general, greater endometrial distortion more clearly results in compromised fertility. Myomectomy improves fertility in these cases.
There is increasing data that suggests that intramural fibroids reduce fertility, especially when intramural myomas are 4 cm or larger, and myomectomy appears to restore fertility [10]. Some studies have found that fibroids between 2 and 4 cm may also compromise fertility [11]. However, the data is far from conclusive. A recent meta-analysis found a nonsignificant trend of slightly lower pregnancy rates in women with intramural myomas and a nonsignificant trend of improved pregnancy rates after myomectomy in women during assisted reproduction procedures [12]. At this time, there is no clearly proven benefit of myomectomy to enhance fertility for women with intramural myomas [13, 14]. Subserosal fibroids do not appear to affect fertility [15].
Many women have multiple fibroids, and the different size, location, number, and relative relationship to the endometrium increases the difficulty in establishing the effect of fibroids on fertility, since it is likely that no two individuals are directly comparable (Fig. 10.5). As such, the relative usefulness of myomectomy in these situations cannot be established with certainty.
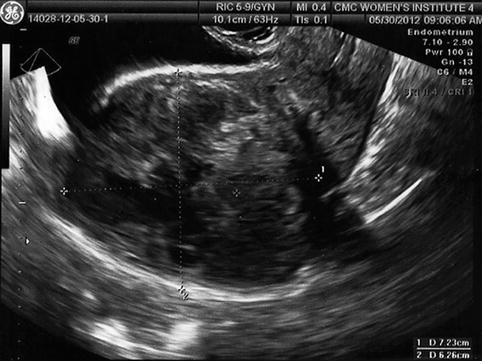

Fig. 10.5
Transvaginal ultrasound demonstrating multiple intramural fibroids; the entire endometrium is difficult to visualize
Fibroids and IVF
Studies of the impact of fibroids in IVF cycles is helpful to establish the impact, since many factors impacting fertility are either controlled, such as male infertility, or directly evaluated, such as the impact of age on cycle outcome. Submucosal fibroids clearly reduce IVF pregnancy and birth rates [16, 17]. Furthermore, hysteroscopic myomectomy significantly improves pregnancy rates, with outcomes comparable to women with a normal uterine cavity [18].
The effect of medium and large intramural myomas on IVF outcomes is unclear, and most studies have shown little clinical effect. One retrospective study showed that IVF live birth rates were not improved by myomectomy: IVF “ongoing” pregnancy rates were 17 % after myomectomy (n = 47), 21 % with untreated fibroids (n = 11) and 19 % in normal controls [17]. However, 50 % of women with fibroids experienced a spontaneous abortion, compared to 34 % after myomectomy, possibly suggesting that fibroids compromise pregnancy outcomes. Another study of 46 IVF with intramural and subserosal fibroids showed that IVF outcomes were similar to controls, but fibroid size was not assessed [19]. Other investigators found that myomas, 73 % of which were subserosal, had no effect on conception in 39 women [20]. A large recent study found that asymptomatic women with subserosal or intramural fibroids smaller than 5 cm did not compromise IVF pregnancy or birth rates, as long as there was no endometrial distortion [21]. One hundred nineteen women with asymptomatic intramural or subserosal fibroids were matched to controls by age and number of previous IVF cycles. The outcome was not changed when the group was limited to those with intramural myomas.
Contrary to these reports, some studies have shown that intramural fibroids impair IVF outcomes. A retrospective study found a significant decrease in IVF live birth rates in women under age 40 years with intramural fibroids (49 and 58 %, respectively) [22]. In 2005, a meta-analysis showed a significantly lower implantation rate with intramural fibroids compared to controls, 16.4 % vs. 27.7 %, respectively (OR 0.62, 0.48–0.8), and a significantly lower birth rate per embryo transfer with fibroids compared to controls, 31.2 and. 40.9 % (OR 0.69, 0.50–0.95) [23]. In a retrospective study of 91 IVF cycles in women with intramural or subserosal fibroids, Stovall et al. found a significantly lower pregnancy rate with fibroids (37 %) compared to matched controls (53 %) [24]. The fibroids’ size ranged from 8 to 54 mm, with a mean diameter of 29 mm, and 95 % were intramural. The Implantation rate was 14 % with fibroids, significantly lower than the 20 % implantation rate in controls without fibroids. Another study found that women with intramural fibroids had significantly lower pregnancy rates compared to women without fibroids, 16 and 34 %, respectively, p <0.05 [16]. Implantation rates were more than 50 % lower with intramural fibroids compared to the controls (p < 0.005), even though the mean diameter of the fibroids was 24 mm. A meta-analysis assessed 19 observational studies comprising 6,087 IVF cycles and found a significantly lower IVF live birth (RR = 0.79, 95 % CI: 0.70–0.88, p < 0.0001) and clinical pregnancy rate (RR = 0.85, 95 % CI: 0.77–0.94, p = 0.002) in women with intramural fibroids compared to those without fibroids [25]. The authors concluded that non-cavity-distorting intramural fibroids are associated with adverse pregnancy outcomes in women undergoing IVF. Finally, a study by Oliveira et al. found a significantly lower pregnancy rate with IVF only when intramural fibroids were 4 cm or larger [10].
Egg donation provides an opportunity to study the effect of implantation while minimizing the effect of confounding factors of maternal age and male fertility. There is evidence that egg donation outcomes are lower in African-American women compared to other populations, although the populations are too small to conclude that fibroids are the primary explanation for this effect [26]. It is possible that uterine fibroids could provide a possible explanation for this observation.
Uterine fibroids may increase the difficulty of the oocyte retrieval or embryo transfer and either may lower IVF outcomes. A fibroid may raise the ovary out of the pelvis, especially large masses. If this occurs, it may be necessary to perform laparoscopic oocyte retrieval or ultrasound-directed transabdominal retrieval. A fibroid may increase the difficulty of the embryo transfer in one of several ways: distorting the position of the cervix in a way that it is difficult or impossible to expose the cervix with a speculum, by markedly altering the endocervical course or causing endocervical stenosis (Fig. 10.6). Finally, a large fibroid may make visualization of embryo transfer difficult or impossible when an abdominal ultrasound-guided procedure is performed. This can be critical, especially for uterine fibroids, since increasing difficulty or tortuosity of the endocervix makes it difficult to visualize the transfer catheter to confirm optimal placement.
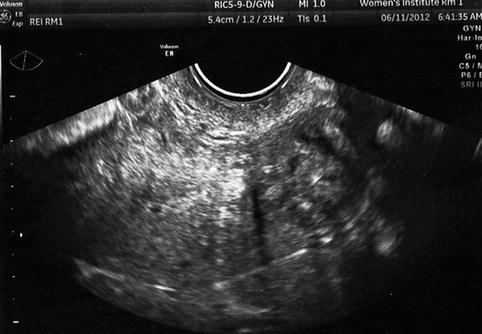

Fig. 10.6
Transvaginal ultrasound demonstrating large fibroid in the lower uterus and cervix
Myomas and Obstetrical Outcomes
While the impact of fibroids on fertility is still debated, obstetrical outcomes appear to be compromised by uterine fibroids [9]. A population-based retrospective study by Sheiner et al. [27] found that women with fibroids had a 3.5-fold increased incidence of intrauterine growth restriction (6.8 % vs. 1.9 %), a fourfold increase in placental abruption (2.8 % vs. 0.7 %), a fivefold higher incidence of transverse lie or breech presentation (16.9 % vs. 2.4 %), a five times higher cesarean section rate (57.7 % vs. 10.8 %), 70 % higher risk of premature rupture of membranes (9.6 % vs. 5.5 %), and were three times more likely to receive transfusion (4.2 % vs. 1.4 %). All of these outcomes were significant, with p < 0.001. Adjusting for maternal age, parity, gestational age, and malpresentation, pregnancies with fibroids still had a 6.7-times higher risk of cesarean delivery, with 95 % CI 5.5–8.1, p < 0.01). Placental abruption and preterm deliveries remained significantly more common with fibroids. The size and locations of the fibroids were not assessed in this study, but other investigators have found that fibroids adjacent to the placenta increase the risk of bleeding and premature rupture of membranes [28].
A retrospective study in 2012 supports the hypothesis that fibroids have a detrimental impact on pregnancy, especially when the fibroids are large [29]. The mean gestation age at delivery for women with fibroids larger than 5 cm was 36.5 weeks, significantly earlier than women with smaller fibroids or no fibroids. Other significant effects included shortened cervix, premature preterm rupture of membranes, preterm delivery, blood loss during delivery, and the need for postpartum transfusion. Considering these and other publications, authors of a literature review concluded that pregnancy outcomes are compromised in women who have intramural fibroids [30].
Uterine fibroids tend to enlarge during pregnancy, regardless of size and maternal age [31]. Approximately 70 % of fibroids grow by a volume of 10 % or more between the first and second, and second and third trimesters. As a fibroid grows, masses located near the cavity have the potential to compress the cavity and cause a detrimental effect.
It would appear that myomectomy could be justified in some circumstances to reduce the risk of adverse pregnancy outcomes [32]. Unfortunately, the benefit of myomectomy for intramural fibroids has not been definitively proven. The most compelling evidence for intramural myomas appears to be cases with large fibroids, 4 cm or larger, and tumors close to the uterine cavity. It is important to clarify this issue since myomectomy for intramural fibroids has a risk of morbidity and adhesion formation, and surgery should not be considered unless the benefits outweigh the risks.
In spite of the limited data, myoma size, location, and number are key factors when discussing the outcomes after myomectomy, and these are considered separately in this discussion. However, these factors are not separable for an individual patient, and the surgeon must weigh the cumulative impact of all three factors when deciding to perform how and when to perform a myomectomy.
Diagnosis of Uterine Fibroids
A focused history and physical examination may provide suspicion of uterine fibroids. Symptoms related to fibroids may include menorrhagia, dysmenorrhea, menstrual clotting, intermenstrual bleeding, pelvic pain, pressure, progressive constipation or alternating constipation and diarrhea, abdominal distention, or urinary frequency. However, other conditions can cause any of these symptoms, and fibroids are often asymptomatic. On examination, the uterus is often enlarged and irregular with uterine fibroids due to the distortion from the individual masses. A rectovaginal examination may be helpful to identify posterior fibroids. However, other conditions such as adenomyosis can cause uterine enlargement, and a clinically significant fibroid may be present, even if the examination is normal. Diagnostic testing with ultrasound is appropriate for any women with infertility and is considered an important component of the infertility evaluation.
Ultrasound
Transvaginal ultrasound provides better image quality than abdominal ultrasound, but both methods might be necessary in the uterus that is markedly enlarged with uterine fibroids. Since overlying bowel may limit the visualization of the uterus, abdominal ultrasound is performed with the bladder full enough to provide a “window” for the uterus. Vaginal ultrasound studies are performed with an empty bladder for patient comfort.
Careful examination of the endometrium and myometrium is needed to assess anatomic abnormalities. A submucous myoma is easily identified when the endometrium has a preovulatory “triple stripe” pattern. If there is no endometrial distortion or deflection of trilaminar endometrium, a submucous fibroid is unlikely. In the early follicular phase and after ovulation, when the endometrium is more homogeneous, endometrial distortion is more difficult to assess and saline infusion sonohysterography should be performed if a submucous fibroid is suspected [33].
Uterine fibroids may appear to have several variations in appearance using ultrasound, depending on the characteristics of the mass. For example, a calcified myoma has a bright echogenic pattern and distortion or “artifact” beyond the mass (Fig. 10.5). Although calcified fibroids are easily identified, distortion that occurs beyond the mass may “hide” the endometrium or other fibroids. Uterine fibroids are sometimes visible as “hypoechogenic” oval masses in the myometrium. Less often, a fibroid may have the same echogenic pattern as the surrounding myometrium and be identified by finding a deflection of the endometrial or the serosal surface of the uterus. Subtle or uncertain findings should be cautiously interpreted, since normal physiologic contractions of the myometrium can be confused with an intramural fibroid. If an uncertain abnormality is suspected, the sonographer should reassess the area of interest after a few minutes to allow a contracted area to change. Another method that may be helpful is the use of color Doppler. Since the fibroid is surrounded by a rich vascular supply, a myoma will usually demonstrate a “ring of fire” [34]. In some cases, 3-D ultrasound may also provide additional anatomic insight regarding the position of the myoma.
Fibroids must be differentiated from adenomyosis, especially when surgery is considered, since resection of adenomyosis and repair of the defect can be difficult [33]. Adenomyosis may have several appearances by ultrasound, making the diagnosis uncertain in some cases. To add to the confusion, hormonal changes might cause variations in the appearance of an area of adenomyosis throughout the cycle, since the response of adenomyosis is similar to the normal endometrial response. Adenomyosis may appear hyperechoic, hypoechoic, or the signal may be mixed. Adenomyosis can enlarge or shrink throughout a menstrual cycle, depending on the hormonal response. In some cases, adenomyosis forms a nodular myometrial mass which is readily identified by ultrasound. Adenomyosis can also be a diffuse condition affecting a large segment of the myometrium, with the only ultrasound finding being a subtle uterine enlargement. Sometimes, adenomyosis and uterine fibroids have a remarkably similar appearance with ultrasound, and some women have both conditions. Color Doppler studies are helpful to distinguish uterine fibroids from adenomyosis, since vascular flow is peripheral with fibroids, and more homogeneously affects adenomyosis lesions.
Saline Infusion Sonohysterography
Saline infusion sonohysterography (SIS) is a routine procedure performed in preparation for IVF in many centers. SIS is also helpful when a submucous or sessile myoma is suspected based on clinical history or ultrasound examination.
Saline infusion sonography is performed by filling the uterus with saline while assessing the uterine cavity by transvaginal ultrasound. If evaluation of tubal patency is needed, a bubble/saline infusion system (FemView, Femasys, Suwanee, Georgia) may be beneficial [35].
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a highly sensitive method to define the size, number, and location of fibroids. While the expense of the test limits the widespread utilization, in some circumstances when myomectomy is planned, MRI can help determine whether abdominal or laparoscopic myomectomy is the more appropriate route. MRI can also be useful to visualize an extremely large uterus, whereas the field of visualization is limited with ultrasound. MRI is beneficial to visualize the uterus when calcifications make adequate assessment difficult with transvaginal or abdominal ultrasound. Finally, MRI can help differentiate fibroids and adenomyosis [36].
Management of Uterine Fibroids
Observation
Observation is reasonable for infertile women who are asymptomatic, especially with intramural fibroids smaller than 4 cm. Furthermore, observation is appropriate for symptomatic infertile women with subserosal or pedunculated fibroids, as long as the severity of symptoms does not warrant surgery.
There is no evidence that medical therapy of uterine fibroids enhances fertility. However, medical therapies can help prepare the endometrium to provide optimal visualization of hysteroscopy and to correct anemia before surgery.
Medical therapies for fibroids have included hormonal contraception and GnRH agonists. Except for the use of the levonorgestrel IUD to reduce bleeding in women with small submucosal fibroids [37], there is limited evidence for efficacy in controlling excessive bleeding, especially when fibroids distort the uterine cavity [38]. Pre-hysteroscopy GNRH agonists can be used to decrease preoperative menorrhagia, to allow for recovery of anemia, and to thin the endometrium in preparation for hysteroscopic myomectomy, although preoperative GNRH agonists can increase the difficulty in enucleating fibroids during myomectomy, increasing the difficulty of the procedure [39]. The high cost, hypoestrogenic side effects, and limited efficacy limit the long-term use of GnRH agonists for infertile women with uterine fibroids.
Surgery
Indications for myomectomy for infertile women with uterine fibroids include (1) abnormal uterine bleeding not responding to conservative treatments, (2) high level of suspicion of pelvic malignancy, (3) growth after menopause, (4) infertility when there is distortion of the endometrial cavity or tubal obstruction, (5) recurrent pregnancy loss (with distortion of the endometrial cavity), (6) pain or pressure symptoms that interfere with quality of life), (7) urinary tract symptoms (frequency and/or obstruction), and (8) iron deficiency anemia secondary to chronic blood loss [40]. However, in women with otherwise unexplained infertility, myomectomy may improve pregnancy outcomes [39, 41]. Therefore, surgery should be recommended for infertile women with submucous myomas and considered for women with a myoma, 4 cm or larger, or intramural myomas within a few millimeters of the endometrial cavity in women with otherwise unexplained infertility when appropriate fertility treatments have been unsuccessful.
Myomectomy is usually the best option for young women who desire preservation of the uterus. The type of myomectomy, hysteroscopic, open, or laparoscopic, is chosen based on patient symptoms, location, size and number of fibroids, and the skill and experience of the surgeon. Considerations to the route of surgery must include selection of the approach that provides greatest improvement in the prognosis, a high safety profile. The decision should be made with the knowledge of the length of patient recovery and cost of the procedure, but these should not be the deciding factors for choosing the surgical route.
An appropriate preoperative evaluation is essential to prepare for surgery. In many cases, ultrasound establishes the fibroid size and location. SIS is beneficial to determine the relationship of a submucous myoma to the myometrium for women with a submucosal myoma. MRI should be considered if an atypical ultrasound appearance is identified, if the ultrasound is not conclusive, or if adenomyosis is suspected.
Hysteroscopic Myomectomy
Hysteroscopic myomectomy is the most appropriate approach when a submucous myoma has been identified, before initiating fertility treatment. Hysteroscopic myomectomy is appropriate for symptomatic submucous myomas and for infertile women with asymptomatic submucous myomas.
Reproductive outcomes are improved following hysteroscopic myomectomy [9]. Hysteroscopic myomectomy lowers the incidence of first trimester losses and improves the term live birth rate [42]. In women with menorrhagia caused by a submucous myoma who desire fertility preservation, hysteroscopic myomectomy compares favorably to hysterectomy [43].
For optimal visualization during hysteroscopy, the procedure is either performed in the follicular phase of the cycle to ensure a thin endometrial lining or after pretreatment with a GNRH agonist or hormonal contraception for 1 or 2 months before surgery. Hysteroscopic myomectomy can be performed concurrently with abdominal or laparoscopic myomectomy to reduce bulk symptoms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree