By harnessing the versatility and soft tissue imaging capabilities of MR imaging alongside the unmatched sensitivity and biomolecular flexibility of PET, the potential to provide detailed multiparametric plaque characterization in the carotid arteries is clear. The ability to acquire simultaneous, and dynamic multimodal data is perhaps PET/MR’s greatest strength that will be of major interest to researchers investigating carotid and coronary atherosclerosis alike. This review summarizes the current status of dedicated hybrid PET/MR imaging; to crystallize the rationale for and advantages of this technique with respect to carotid atherosclerosis; and to discuss current limitations, challenges, and future directions.
Key points
- •
By harnessing the versatility and soft tissue imaging capabilities of MR imaging alongside the unmatched sensitivity and biomolecular flexibility of PET, the potential to provide detailed multiparametric plaque characterization in the carotid arteries is clear.
- •
Both these modalities already have a proven track record in vascular imaging and the capability to cross validate one against the other is attractive, particularly in an era in which obtaining good tissue for image validation is becoming more difficult.
- •
The ability to acquire simultaneous, and dynamic multimodal data is perhaps PET/MR’s greatest strength that will be of major interest to researchers investigating carotid and coronary atherosclerosis alike.
Introduction
Stroke remains a major global cause of morbidity and mortality, while controversy and debate as to the best method for imaging and treating carotid atherosclerosis endures. Anatomic measures of carotid luminal stenoses remain the imaging gold standard and are widely used to risk stratify patients and guide invasive management. Carotid stenosis is conceptually straightforward and measured easily, cheaply, and reliably on a variety of platforms; yet, it is also profoundly limited as a biomarker, as we know that most patients even with a high-grade (70% to 99% by the criteria of the North American Symptomatic Carotid Endarterectomy Trial Collaborators ) symptomatic anatomic stenosis will not go on to have further events. With this problem in mind, an expert group has described the search for reliable molecular imaging of the extracranial carotid arteries as “critical.” Unfortunately it has proven challenging to identify such a technique capable of improving the current paradigm.
Imaging the carotids is not just about identifying the high-risk plaque or patient but also concerns measuring and monitoring pathologic disease burden and activity. This can provide not only mechanistic insight but also a method for monitoring response to therapy and for testing the biological plausibility of novel treatments. In the cardiovascular field, ultrasound, computed tomography (CT), MR imaging, and more recently PET have all demonstrated promise in these respects and in several instances been used successfully in interventional trials. Each has their own individual strengths and limitations, leading to major interest in the use of hybrid imaging systems capable of harnessing the information provided by 2 or more approaches. In this regard, PET/CT has for many years led the field; however, more recently hybrid PET/MR systems have been brought to market with the intention of combining PET with the advantages that MR imaging holds over CT, principally the improved soft tissue contrast and the lack of radiation exposure.
The aim of this review was to summarize the current status of dedicated hybrid PET/MR imaging; to crystallize the rationale for and advantages of this technique with respect to carotid atherosclerosis; and to discuss current limitations, challenges, and future directions.
Introduction
Stroke remains a major global cause of morbidity and mortality, while controversy and debate as to the best method for imaging and treating carotid atherosclerosis endures. Anatomic measures of carotid luminal stenoses remain the imaging gold standard and are widely used to risk stratify patients and guide invasive management. Carotid stenosis is conceptually straightforward and measured easily, cheaply, and reliably on a variety of platforms; yet, it is also profoundly limited as a biomarker, as we know that most patients even with a high-grade (70% to 99% by the criteria of the North American Symptomatic Carotid Endarterectomy Trial Collaborators ) symptomatic anatomic stenosis will not go on to have further events. With this problem in mind, an expert group has described the search for reliable molecular imaging of the extracranial carotid arteries as “critical.” Unfortunately it has proven challenging to identify such a technique capable of improving the current paradigm.
Imaging the carotids is not just about identifying the high-risk plaque or patient but also concerns measuring and monitoring pathologic disease burden and activity. This can provide not only mechanistic insight but also a method for monitoring response to therapy and for testing the biological plausibility of novel treatments. In the cardiovascular field, ultrasound, computed tomography (CT), MR imaging, and more recently PET have all demonstrated promise in these respects and in several instances been used successfully in interventional trials. Each has their own individual strengths and limitations, leading to major interest in the use of hybrid imaging systems capable of harnessing the information provided by 2 or more approaches. In this regard, PET/CT has for many years led the field; however, more recently hybrid PET/MR systems have been brought to market with the intention of combining PET with the advantages that MR imaging holds over CT, principally the improved soft tissue contrast and the lack of radiation exposure.
The aim of this review was to summarize the current status of dedicated hybrid PET/MR imaging; to crystallize the rationale for and advantages of this technique with respect to carotid atherosclerosis; and to discuss current limitations, challenges, and future directions.
Currently available platforms, technical considerations, and feasibility
Fully integrated hybrid PET/MR systems are a compromise, with the MR component of the scanner necessitating alteration of the PET component and vice versa. PET detectors were not originally conceived to function in strong magnetic fields and photomultiplier tubes cannot operate in such an environment. Initial approaches to hybrid PET/MR imaging therefore simply consisted of attempting to fuse images acquired separately on different PET/CT and MR scanners. This technique can work well in nondeformable regions of anatomy, such as the skull, in which rigid registration can take place off-line; however, it is less straightforward in soft tissue structures, particularly ones that are mobile. To advance the field, different manufacturers have taken different approaches: the first systems maintained separation of the MR imaging and PET components (eg, Philips Ingenuity TF PET/MR [Phillips, The Netherlands]) but used a dedicated rail-based shuttle table to transfer the patient between scanners in an attempt to minimize movement and facilitate coregistration. The first ever installed worldwide was in 2009 at Mount Sinai Medical Center in New York. However, in practice this approach was suboptimal and lacked the advantages associated with a truly synchronous imaging system.
More recently, genuinely integrated hybrid scanners have come to market ( Fig. 1 ). Incorporating significant alterations to both the MR and PET components of these systems has allowed both imaging modalities to be combined into a single gantry. The Siemens Biograph mMR device (Siemens, Erlangen Germany) uses an avalanche photodiode detector, whereas the more recently available GE system (General Electric, New York, USA) (Signa PET/MR) uses a silicon-based photomultiplier tube, both of which are capable of functioning within a magnetic field and therefore allow the simultaneous acquisition of PET and MR imaging data. The challenges of adequately shielding the PET components without negatively impacting on MR quality have been met principally thanks to developments in gradient design, making increasing bore sizes possible.
Many studies have now been published in various domains showing that the use of these devices is feasible (for a recent review, refer to Tudisca and colleagues ), but it goes without saying that they are expensive, require a large interdisciplinary support staff, and represent a significant ongoing financial commitment.
Rationale for combining PET with MR for carotid plaque imaging
MR offers multiple key advantages to CT when imaging the carotid arteries, supporting PET/MR as the hybrid system of choice for molecular imaging of these regions. Although previously this was tempered by problems in acquiring and quantifying the PET data, recent technological advances have ensured that solutions to these issues have largely been found. The potential advantages of PET/MR imaging compared with PET/CT fall into several domains ( Table 1 ).
Carotid Angiography
In terms of plaque visualization and segmentation, although CT is able to achieve submillimeter spatial resolution in combination with a very short acquisition time, soft tissue contrast is poor and 1-dimensional (ie, plaque segmentation is based purely on photon attenuation). CT carotid imaging is also hampered by blooming artifact from dense calcification. Both these factors make visualization of the luminal and adventitial borders for region of interest analysis difficult. By comparison, MR with black-blood imaging offers superior soft tissue detail that provides excellent contrast at the adventitial and luminal borders (see Fig. 1 ). Iodinated contrast enhancement does improve visualization on CT but may be contraindicated in renal failure or with previous contrast reactions and does nothing to abrogate blooming from calcium. Although MR angiography with gadolinium remains a concern in patients with advanced renal failure, alternative technique can be performed safely in these patients either via the use of alternative contrast agents (iron oxide nanoparticles ) or using standard time of flight (TOF) MR sequences.
Limitations of Histologic Validation and Cross Validation with MR Imaging
A standard and productive model for cardiovascular PET research has been to scan patients just before carotid endarterectomy (CEA). The excised plaque can then be collected and used to validate the PET radiotracer in question using histologic or even gene expression techniques. This model is, however, becoming difficult to deliver as a result of the increasing impetus to intervene expeditiously on patients with symptomatic carotid stenosis and a greater reluctance to intervene on asymptomatic lesions. A further consideration is that the role of carotid artery stenting may increase, reducing the role of endarterectomy and the opportunity to use this valuable approach. Histologic examination also has its limitations: patients undergoing endarterectomy are invariably at the severe end of the disease spectrum, biasing such studies against the earlier phases of the condition. Moreover, the processing of the heterogeneous and calcified necrotic tissue that emerges from surgery is notoriously difficult, and further complicated by trauma to the specimen at the time of its excision. Finally, histologic sampling is necessarily limited to small regions of the plaque and thus prone to sampling bias. Critically, these regions do not include the outer tunica media and tunica adventitia of the carotid artery (because of the way the plaque is excised), tissues that are increasing recognized to play central roles in atherogenesis.
Alternative methods of validating novel PET radiotracers and imaging techniques are therefore required. Because of the limitations previously discussed, CT is not able to adequately resolve carotid plaque for detailed compositional and phenotypic analysis. By contrast, MR provides much greater information with respect to plaque morphology, with the ability to identify multiple key plaque characteristics, including an intact or ruptured fibrous cap, a necrotic lipid-rich core, intraplaque hemorrhage, thrombus, intraplaque neovascularization, macrophage infiltration, biomechanical stress, and others ( Fig. 2 ) (a recent review by Makris and colleagues is recommended ). Although obviously not as “definitive” as histology, MR is an excellent alternative and complementary technique with the key advantage that plaques can be assessed in situ at multiple stages in their development. MR also permits serial and 3-dimensional volumetric assessment of the carotid artery in its entirety, including the layers missed with histologic validation. On this basis, hybrid PET/MR offers the opportunity to cross validate a wide range of novel PET tracers, providing mechanistic information beyond simple anatomic coregistration ( Table 2 ).
| Process | Target | PET Radiotracer | MR Technique (Molecular or Structural) |
|---|---|---|---|
| Inflammation/cell recruitment | VCAM-1 | — | VCAM-1-BP + USPIO |
| VCAM-1-BP + Gd | |||
| ICAM-1 | — | ICAM-1 ligand + MPIO | |
| Glycolysis | [18F]-FDG | — | |
| TSPO receptor | [11C]-PK11195 | — | |
| Macrophage scavenger receptor | [124I]-CD86-Fc | SR-A1 ligand + USPIO | |
| Somatostatin Receptor 2 | [68Ga]-DOTATATE | — | |
| Phagocytosis | — | Unconjugated USPIO | |
| — | Emulsified perfluorocarbons | ||
| Matrix and proteinases | MMP | Various | P947 + Gd |
| Elastin | — | BMS753951 + Gd | |
| Lipid-rich core | Lipid pool | — | LDL particles + Gd |
| Hypoxic cells | [18F]-FMISO | — | |
| [18F]-FDG | — | ||
| Cell death | Phosphatidylserine | — | Phosphatidylserine targeting peptide + Gd |
| Caspase 3 activity | [ 18 F]-CP18 | ||
| Angiogenesis | α ν β 3 -integrins | [18F]-galacto-RGD | RGD-peptide mimetic + Gd |
| Neovessels | — | DCE-MR imaging | |
| Intraplaque hemorrhage | Methemoglobin | — | Multicontrast MR imaging |
| Calcification process | Active micro-calcification | [18F]-Fluoride | — |
| Thrombosis | Fibrin | [64Cu]-EP-2104R | EP-2104R + Gd |
| [64Cu]-FPB7 | — |
Indeed, the feasibility of this approach has already been demonstrated in several clinical studies using sequentially acquired [18F]-FDG PET/CT and MR, studies that would have been greatly simplified by a hybrid system. Moreover, 2 elegant preclinical studies have recently been published in this domain, the first cross-validating a fibrin-targeted probe (EP-2104R) and the second comparing ultrasmall superparamagnetic iron oxide–enhanced MR with [18F]-FDG as alternative markers of plaque inflammation. The availability of such preclinical PET/MR systems, capable of scanning human specimens ex vivo and small animals in vivo, is likely to accelerate the development and clinical translation of this imaging technique.
Simultaneous Scanning and Motion Correction
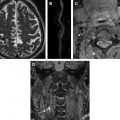
CT and PET datasets are necessarily acquired sequentially and are frequently either degraded if not frankly spoiled by movement. The nature of this movement can either be intrinsic (ie, cardiac motion, respiratory motion, swallowing, hollow viscus peristalsis) or extrinsic (ie, an uncomfortable patient who squirms or shifts position). The ability to simultaneously acquire PET and MR data is a critical advantage that modern hybrid PET/MR systems hold. This opens up the possibility of accurate motion correction with huge potential benefits for imaging of the coronary arteries and the myocardium in addition to the carotids ( Fig. 3 ).
With PET/CT, the only options for tackling motion relate either to measures aimed at controlling such movement (ie, asking patients to lie still, ensuring their comfort, or using neck collars and blocks to provide gentle restraint) or to postprocessing the PET data in an attempt to correct for it. Postprocessing may involve either registration (manual or automated, rigid or nonrigid) that is limited by poor PET spatial resolution (∼4.5–5 mm) and a lack of fiducial landmarks or, in the case of cardiac and respiratory motion, retrospective gating. Retrospective gating is accomplished by acquiring PET data in list mode (analogous to using a video camera instead of a photographic camera) and simultaneously acquiring electrocardiogram and respiratory cycle data. This permits retrospective exclusion of PET data acquired during portions of the cardiac and/or respiratory cycle where the region of interest is unacceptably mobile. Unfortunately, discarding most PET events with retrospective gating has a major negative impact on the signal-to-noise ratio (SNR) that can presently only be abrogated by increasing radiotracer dose or patient bed-times. Additionally, this retrospective approach does not typically attempt to correct for the effects of patient motion when constructing the attenuation correction maps, leading to the potential for systematic biases in PET quantification.
Hybrid PET/MR can permit improved retrospective gating either by directly measuring organ location (eg, by using a diaphragmatic navigator) or far more elegantly, by obtaining continuous and synchronous MR and PET data. Indeed, the latter approach offers the theoretic and enticing possibility of correcting for both intrinsic and extrinsic movement without having to discard any emission data. Not only would this significantly enhance image quality by minimizing motion blur and improving SNR, it would also have major cost implications, as scanning times could be reduced allowing more patients to be scanned in a given scanning session or production run on the cyclotron. Ultimately, such economic factors could prove crucial in translating cardiovascular PET imaging into the clinical environment.
Robust motion correction using PET/MR might also make accurate dynamic PET imaging of moving tissues a possibility. The general principle of MR-based motion correction involves recording the displacements of the relevant anatomy in space and time (ie, in 4 dimensions) using MR and then using these data to either retrospectively “deconvolve” the reconstructed and time-binned PET data, or better still, to achieve this prospectively by including the 4-dimensional MR data in the reconstruction algorithm. In principle, the same methods also may be used to reduce the impact of movement on attenuation correction, thus further improving data quality. It is beyond the scope of this review to discuss these methods in detail but the reader is referred to excellent articles by Catana and Petibon and colleagues. Although the biggest impact of these advances may well apply to PET imaging of the heart, such motion correction also will be of direct relevance to carotid imaging. Indeed, little can presently be done to control for small extrinsic movements or the effects of swallowing and jaw movement, which can have a major impact on PET quantification in small-volume carotid plaques.
Radiation Exposure, Serial Imaging, and Multiprocess Imaging
One of the major attractions of PET is that it is able to resolve specific biological pathways and physiologic activity in vivo with exquisite sensitivity and without disturbing the subjects’ biology. This had led to a new model for phase 2 clinical trials in which novel pharmacologic agents are tested for biological efficacy in vivo using serial PET examinations, before proceeding to large and expensive phase 3 trials. Indeed, we successfully used this approach in the recent dal-PLAQUE study and are currently conducting several other similar studies in our institutions. One of the major concerns relating to this approach is the large cumulative dose of radiation that may accrue with multiple PET/CT examinations. The ability to remove CT from the equation will go a long way to allaying anxiety on this front, and in opening up the possibility of examining disease activity at 3 or 4 different time points, or investigating several disease processes simultaneously.
Potential for Multiprocess Imaging and PET Sensitivity and Specificity
As well as permitting cross validation, PET/MR offers the opportunity to combine the functional information of PET with emerging molecular MR techniques, rendering true multimodality, multiprocess imaging possible. This would allow for studies, incorporating a wide array of both PET and MR techniques, capable of investigating multiple pathologic processes simultaneously. A complete discussion of emerging molecular MR techniques is beyond the scope of this review, but includes the design of specific molecular MR tracers incorporating either paramagnetic (gadolinium) or superparamagnetic (coated iron oxide nanoparticles) reporters (see Table 2 ), dual-tuned coils to enable simultaneous [1H] and [19F] imaging of perfluorocarbon tracers, MR spectroscopy, and hyperpolarized MR (indeed, the concept of “HyperPET” has already entered the lexicon ). The opportunity also exists to design imaging nanoparticles with reporters for both PET and MR. Although promising, several of these MR techniques are currently limited either by the nonlinearity of reporter accumulation and signal change or by low sensitivity. Indeed, PET is generally quoted to be 3 to 4 orders of magnitude more sensitive than MR, which of course is a key attraction of PET.
Versatility and Concurrent Imaging of Other Relevant Organ Systems
MR imaging is currently the gold standard technique for imaging the brain, and many of the radiotracers of interest in the carotid also will be of interest in the brain (eg, [18F]-FDG). The ability to do a full simultaneous vasculo-cerebral PET/MR assessment using multiple complementary sequences (eg, diffusion-weighted imaging abnormalities or cerebral perfusion imaging in the context of a carotid stenosis) and PET acquisitions will therefore be of major interest to stroke and vasculopathy researchers, especially as it will not involve additional radiation exposure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree