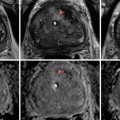
Fig. 28.1
A 19-year-old male with left-sided varicocele. (a) The gray-scale sonography shows dilated veins in the pampiniform plexus measuring 5 mm. (b, c) The color Doppler sonography demonstrates reflux during the Valsalva maneuver indicated by the increase in color flow
Treatment
Varicocele treatment is indicated in patients with clinical varicocele with (1) male infertility and with abnormal semen parameters or function test, (2) greater than 20 % testicular volume differential that persists for more than a year in the pediatric population, (3) scrotal discomfort or pain, or (4) hypogonadism [3, 24–27].
Varicocelectomy
Basic surgical approaches in ligating the internal spermatic vein include retroperitoneal, inguinal, and subinguinal. Various varicocele ligation techniques have been developed over the past decades. Open (Palomo) or laparoscopic retroperitoneal, macroscopic inguinal (Ivanissevich), and microscopic subinguinal techniques are among the many available options [4, 28, 29]. For the high retroperitoneal approach, a transverse incision is made medial and inferior to the anterior superior iliac spine at the level of the inguinal ring. By incising the external oblique fascia and retracting the internal oblique muscle, the internal spermatic veins are exposed, dissected, and ligated. Doppler probe is typically used to facilitate the identification of the testicular artery. Laparoscopic varicocelectomy is generally performed transperitoneally, while retroperitoneal and extraperitoneal approaches have been described. Laparoscopic technique provides higher magnification and is performed nearer the left renal vein where there are fewer large draining gonadal veins, far from the takeoff of the testicular artery.
For the inguinal approach, the incision is made just above the pubic symphysis. Incision of the external oblique fascia allows mobilization of the spermatic cord, and the internal spermatic veins are exposed and ligated after the spermatic fascia is incised.
Microsurgical subinguinal varicocelectomy is the newest technique that has gained popularity among the male infertility specialists because of its favorable outcomes. For the subinguinal approach, an incision is made at the external inguinal ring avoiding division of the abdominal fascia and muscle. Use of 8–15 power optical magnification and microvascular Doppler allows clear identification of vessels and lymphatics minimizing the risk of accidental ligation.
Recent meta-analysis by Cayan et al. has reported overall spontaneous pregnancy in 39 % of 4473 subfertile or infertile men undergoing varicocele treatment with various techniques. The highest spontaneous pregnancy rate of 39 % was achieved after varicocele repair [30]. The highest spontaneous pregnancy rate of 42 % was achieved in the microscopic subinguinal technique, compared to 38 % in the Palomo, 30 % in the laparoscopic, and 36 % in the macroscopic inguinal techniques. The lowest recurrence rate of 1 % was seen in the microscopic subinguinal technique while 15, 4.3, and 2.6 % recurrence rates were seen in the Palomo, laparoscopic, and Ivanissevich techniques, respectively. The lowest hydrocele formation rate of 0.4 % was reported in the microscopic subinguinal technique, compared to 8, 2.8, and 7 % in the Palomo, laparoscopic, and macroscopic inguinal techniques. Rare complications included testicular atrophy after incidental artery ligation and intraperitoneal injury to bowel, bladder, or neurovascular bundles.
Percutaneous Embolization
Retrograde gonadal vein embolization is the standard procedure for primary varicocele treatment or salvage therapy for recurrent varicocele. Retrospective study of varicocele treatment with surgical ligation and percutaneous embolization by Shlansky-Goldberg et al. has reported comparable improvement in seminal parameters and pregnancy rates of 39 and 34 % after embolization and surgery, respectively [31]. Advantages of percutaneous varicocele embolization include anatomic delineation of the venous collaterals and sparing of injury to the lymphatic drainage and testicular artery. While hydrocele formation is a major postoperative complication after surgical ligation, it has never been reported after embolization.
Embolization is generally performed via a right femoral vein approach. A 5–6 French Cobra catheter is typically used to catheterize the left renal vein then the orifice of the left gonadal vein. Use of a coaxial system with a 6–7 French preformed long sheath or guiding catheter provides additional stability. Use of a microcatheter facilitates the catheterization of the small caliber veins with less risk of vasospasm or perforation. The origin of the right gonadal vein typically drains into the right anterolateral aspect of the IVC inferior to the right renal vein. A sidewinder-type catheter like a Mickelson, Sos Omni, or Simmons catheter is usually used on the right. Given the size of most IVCs, a catheter with larger secondary curve may aid in access due to its reverse curvature and larger secondary curve which can brace itself against the IVC wall. Internal jugular or basilic vein approach can be used in the setting of unsuccessful catheterization of the right gonadal vein via the femoral approach.
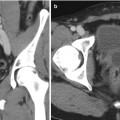
After catheterizing the gonadal vein, venography is performed with the patient placed in the reverse Trendelenburg position. Venograms are obtained at various levels while advancing the catheter from the orifice of the gonadal vein to the level of the pubic symphysis for mapping of the highly variable venous collateral pathways. In general, the gonadal vein branches into the medial and lateral divisions at the level of L4. While the medial division drains are joined by the periureteral veins to form a venous plexus along the ureter draining into the left renal vein or IVC, the lateral division joins the renal capsular and colonic veins. Cross communication between the left and right spermatic veins has been reported in 55 % of the human cadavers [10]. Duplication of the gonadal vein in the inguinal region and communication with the retroperitoneal veins such as the lumbar, renal capsular, iliac, and rarely circumaortic renal veins are among the common collateral pathways (Fig. 28.2). Incomplete interruption of these collateral pathways contributes to the persistence or recurrence of varicocele after surgical or interventional treatment [32–34].
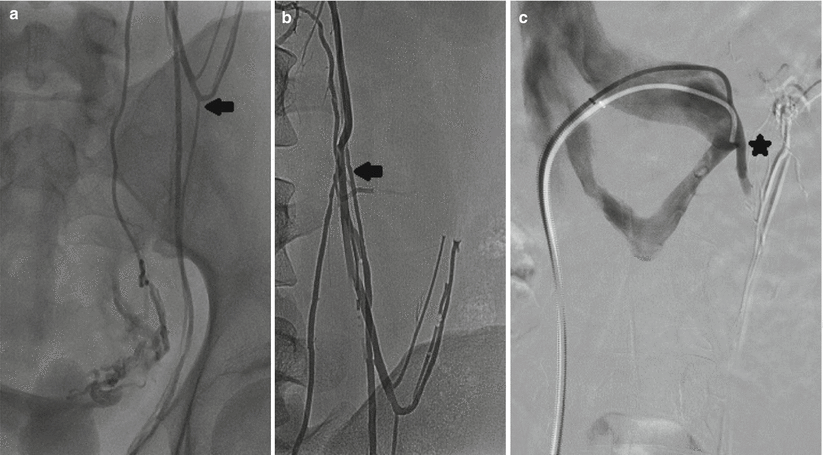

Fig. 28.2
A 24-year-old male with bilateral scrotal pain elicited when lifting weights. (a, b) Duplicated gonadal veins join retroperitoneal veins (arrows) at the level of the upper sacroiliac joint. (c) The left gonadal vein drains into the circumaortic renal vein (asterisk)
Various embolic agents are used in embolization including coils, vascular plugs, and sclerosants. Coils are the most commonly used embolic agents and can be classified based on the mechanisms of deployment and occlusion. A detachable coil has many advantages as its release mechanism allows precise positioning of the coil mass and safe withdrawal prior to deployment, in cases of inappropriate sizing relative to the vein diameter or inaccurate positioning. However, a detachable coil tends to be more costly. While a fibered coil induces thrombosis around the coil mass, a hydrogel-coated coil can expand up to nine times its original volume leading to vessel occlusion [35]. Coil embolization of the gonadal vein is performed by placing a nest of coils at the lowest collateral branch, usually at the level of the inguinal ligament. Microcoils are available if a microcatheter is utilized for catheterization at this level. During the retraction of the catheter toward the renal vein, coils are deployed at multiple levels. Generally, coils are placed at four levels: (1) the inguinal ligament, (2) lower sacroiliac joint, (3) upper sacroiliac joint, and (4) several centimeters below the renal vein insertion. Branching points are embolized in an effort to achieve interruption of the collateral pathways (Fig. 28.3). Amplatzer vascular plugs offer a simple, efficient way to occlude large caliber veins. Technical success rate of 96 % has been reported with spontaneous pregnancy rate of 40 % and statistically significant improvement in the semen parameters [36, 37]. Overall complication rate is 11 % including minor complications, contrast extravasation from vascular perforation, coil migration, allergy to contrast agent, and thrombophlebitis of the pampiniform plexus. Major complication rates are about 1 %.
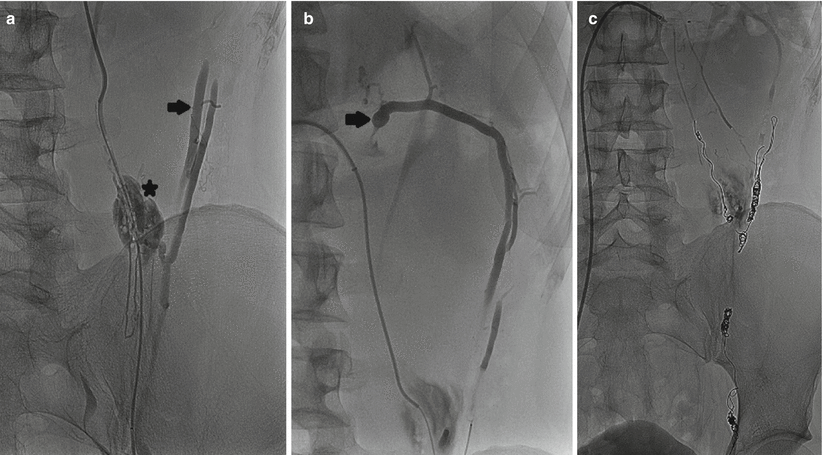

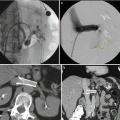
Fig. 28.3
A 25-year-old male with left-sided varicocele and scrotal pain. (a, b) The left gonadal and accessory gonadal veins join the renal capsular veins draining into the left renal vein (arrows). Contrast extravasation is seen at the confluence (asterisk) indicating small perforation. A microcatheter is used to traverse the short segment of venous perforation. (c) Multiple coils are deployed in the gonadal and renal capsular veins
Various sclerosing agents have been used including boiling contrast material, ethanol, sodium tetradecyl sulfate, and sodium morrhuate. Currently, sodium tetradecyl sulfate is the only FDA-approved sclerosant in the United States. Sodium tetradecyl sulfate is typically mixed with air and vigorously agitated between two syringes through a three-way stopcock (Tessari technique) in order to create foam consistency. Upon injection, chemical injury is believed to produce inflammatory reaction leading to endothelial necrosis. A retrospective study by Gandini et al. has reported spontaneous pregnancy rate of 39 % and resolution of testicular pain in 96.5 % of the patients [38]. Similarly, Li et al. have reported technical success rate of 91.4 % with no subsequent recurrence. However, sclerotherapy is often associated with significant inflammatory pain during injection, and reflux of the sclerosant into the undesired venous beds can result in rare complications such as pampiniform thrombophlebitis or renal vein thrombosis.
Coils and sclerosants are often used together in the sandwich technique. After the first nest of coils is deployed at the level of the internal inguinal ring, a sclerosant can be injected on top of the coils. Advantage of this technique is the occlusion of the numerous small collateral channels. In the pediatric population, pain relief and catch-up testicular growth have been reported in 91–94 % of the patients [39, 40].
Pelvic Congestion Syndrome
Chronic pelvic pain is defined as noncyclical pain of at least 6 months duration. Pelvic pain affects approximately one third of all women accounting for 20 % of outpatient gynecologic appointments and numerous diagnostic tests including laparoscopy [41]. The etiology of chronic pelvic pain is varied including gynecologic, urologic, gastrointestinal, neurological, and psychosocial disorders. Pelvic congestion syndrome is characterized by the presence of ovarian and pelvic varicosities in the setting of chronic pelvic pain. Clinical manifestations of pelvic congestion syndrome include pelvic pain exacerbated by prolonged standing, dysmenorrhea, dyspareunia, postcoital ache, and hip pain [42, 43]. Pelvic venous congestion is typically seen in the multiparous women with its prevalence in the general population reaching approximately 10% as reported in the study of healthy female kidney donors undergoing screening renal angiography [44]. However, up to 40 % of asymptomatic women have incidental findings of pelvic varicosities [45, 46]. Hence, pelvic congestion syndrome remains as a diagnosis of exclusion requiring a multidisciplinary evaluation and discussion.
Pelvic congestion is characterized by ovarian venous reflux and dilatation associated with varicosities in the ovarian, uterine, rectal, vesical, vulvar, and/or upper thigh venous systems. The etiology of pelvic congestion is believed to be multifactorial involving mechanical and hormonal factors [47]. Anatomic differences in the left and right ovarian venous drainages, absence of valves in ovarian veins, and markedly increased pelvic venous capacity during late pregnancy are among the proposed mechanical factors leading to venous incompetence and development of pelvic varices. Venous compressions from nutcracker and May-Thurner syndromes are secondary causes of venous engorgement [10–12, 48]. Increased prevalence in multiparous and premenopausal women, presence of polycystic ovaries, and pain relief after administration of progestins or GnRH agonists have been reported, implicating the role of a hyperestrogenic state in pelvic congestion [45, 49, 50]. The cause of pain in pelvic congestion syndrome remains unclear, but pain receptor activation is felt to be the most likely possibility [47, 49].
Diagnosis and Imaging
Pelvic congestion syndrome remains a diagnosis of exclusion after thorough history and physical examination in a multidisciplinary framework. Venography is the “gold standard” because it allows evaluation of the venous reflux and extent of varices in the ovarian, uterine, rectal, vesical, vulvar, and even lower extremity venous systems. Pelvic supply to the varicose veins of the lower extremity has been reported in up to 25 % of women treated for varicose veins [50]. However, venography is not a practical approach due to its invasiveness. Ultrasonography and MRI have gained increasing popularity as initial diagnostic tools because they allow direct visualization of pelvic varices and, more importantly, assess for other pelvic disorders such as endometriosis, uterine myoma, and uterine adenomyosis.
Transabdominal and transvaginal sonographic examination is performed using 2–4 MHz convex and 5–9 MHz intracavitary transducers, respectively, with the patient in the supine position performing the Valsalva maneuver. Dilated ovarian vein with reflux, presence of pelvic varices with dilated cross-uterine arcuate veins, and polycystic changes of the ovary have been reported in pelvic congestion syndrome [51]. Park et al. reported the ovarian vein diameter of 7.9 mm in patients with pelvic congestion syndrome compared to 4.2 mm in the control group. The positive predictive value was 83 % when a cutoff value of the left ovarian diameter was set at 6 mm [52]. However, there is still no uniform criterion on the size of the ovarian vein, and furthermore, some authors have argued that the ovarian diameter alone is not a reliable criterion in the absence of venous reflux [53].
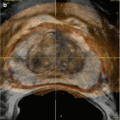
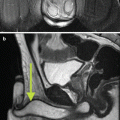
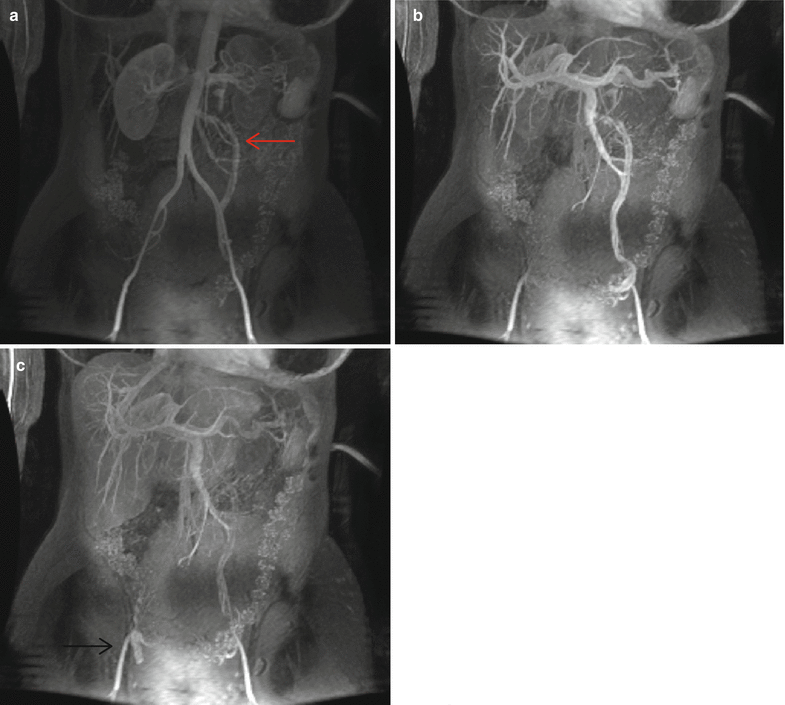
MRI/MRV is the most preferred imaging modality of choice because it allows the anatomic assessment of the abdominopelvic structures without the radiation exposure of CT scan as well as the functional evaluation of the ovarian veins and pelvic varicosities [54]. Varicose veins appear as tortuous, dilated tubular structures around the ovaries and uterus on T1- and T2-weighted images. On dynamic imaging after intravenous administration of the contrast, reflux in the left ovarian vein can be sequentially imaged showing contrast passing through the parametrial and uterine venous plexus to the contralateral side (Fig. 28.4). Using dynamic imaging, diagnosis of nutcracker or May-Thurner syndrome can be reliably made as well.
Get Clinical Tree app for offline access

Fig. 28.4
Time-resolved imaging of contrast kinetics. (a




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree