Many symptoms and signs encountered in the course of clinical care may raise concern for cardiac dysfunction. These manifestations may include chest pain or discomfort, dyspnea, exercise intolerance, edema, syncope, and palpitations. Combined, these complaints account for a substantial share of presentations in both inpatient and outpatient settings. When the clinician strongly suspects a cardiac origin, imaging tests can play a central role in confirming or excluding suspected diagnoses and in selecting among potential therapies or procedures. In some cases, even when other causes may be more likely, imaging tests may be helpful in excluding rarer but potentially life-threatening (i.e., “cannot miss”) cardiac conditions.
Although many different cardiac imaging tests are available to clinicians, each discussed in detail elsewhere in this book, not every test is equally effective in all clinical circumstances and for all questions. Tests have differing strengths, limitations, availability, costs, and contraindications. Subsequently, decision making regarding the most optimal test for a given patient is complex, particularly because no published guidelines are available to use for selecting among the available testing options. Choosing the right test for the right patient requires an understanding of both the clinical question at hand and the technical aspects of the multiple available testing options.
Cardiac imaging tests can be used to evaluate disorders of nearly every aspect of cardiac anatomy and physiology, including estimating cardiac chambers size and function, identifying myocardial scar or infiltration, assessing valvular function, identifying and characterizing cardiac masses, and evaluating the presence and size of pericardial effusions. Because the most frequent indication for cardiac imaging is evaluation of suspected coronary stenosis or ischemia, most of this chapter focuses on this indication. Suspected coronary stenosis or ischemia accounts for a large proportion of cardiac testing, and given the availability of many different testing options, the decision regarding which test to use is most complex.
The purpose of this chapter is to provide a framework for selecting the most optimum test. As such, the discussion answers the following questions:
- 1.
Should a cardiac imaging test be performed?
- 2.
What is the best means to evaluate symptomatic patients with known or suspected CAD?
- a.
What is the best method to determine the pretest probability of disease?
- b.
What are the available testing options?
- c.
What are the advantages of anatomic versus physiologic methods?
- d.
What are the advantages of exercise stress over pharmacologic stress agents?
- e.
When is an imaging test needed in addition to exercise treadmill testing alone?
- a.
- 3.
What is the best means of assessing cardiac risk in asymptomatic individuals?
- 4.
In imaging for noncoronary indications, how does one choose between echocardiography and cardiovascular magnetic resonance (CMR)?
Should a Cardiac Imaging Test be Performed?
Although advances in cardiovascular imaging have greatly improved the ability to diagnose and treat various clinical conditions, the rising cost of testing has generated concern regarding the potential overuse of such procedures. To address these issues, and in recognition of the limited evidence on the clinical effectiveness of imaging, multiple medical specialty societies, including the American College of Cardiology and the American College of Radiology, developed appropriate use criteria (AUC). These criteria, which describe clinical situations in which an imaging test may or may not be appropriate, are available for all the major noninvasive cardiovascular tests. Even though the AUC are intended to facilitate more efficient allocation of health care resources in cardiovascular imaging, they have notable limitations. First, the AUC approach is primarily opinion based because of the paucity of data linking cardiovascular imaging with patient outcomes. Second, some reports have shown that the use of AUC does not lead to a consistent decline in the use of tests deemed inappropriate. Third, the AUC for each imaging modality were developed separately, and as such, they cannot be used for choosing the best test among the numerous testing options that may be available for a given patient. Indeed, for any given patient with a clinical question, several different testing options may be considered appropriate. The American College of Radiology’s AUC use an approach in which a clinical situation (e.g., suspected congenital heart disease in the adult) is discussed and various imaging modalities are rated according to their potential appropriateness. However, the clinical situations are broad categories and are not further refined, and the imaging tests are not weighted against each other or against nonimaging tests.
In deciding whether to obtain a cardiac imaging test for a particular patient, the first step is to define the clinical question at hand ( Box 10-1 ). Next, clinicians must decide whether the test has the potential to alter subsequent patient management (e.g., initiation of a new therapy, referral for further testing or procedures). Tests that confirm a known diagnosis or that identify a condition for which no changes in treatment are anticipated may not be necessary. A more challenging question is this: How likely is an imaging test to provide new information or to answer the clinical question at hand? In some instances, this may be difficult to determine, and a consultation between clinicians and imaging experts may be beneficial for deciding whether further testing would be beneficial.
Diagnosis
- •
Determine the cause of the patient’s symptoms.
- •
Exclude a cardiac condition as a potential cause of the symptoms.
Prognosis
- •
Establish the prognosis (i.e., risk of future cardiovascular events or death).
Treatment
- •
Determine the need, intensity, and type of therapy.
- •
Determine the potential benefit of cardiac procedures.
- •
Evaluate the response to therapy.
What is the Best Means to Evaluate Symptomatic Patients with Suspected Coronary Artery Disease?
In most patients with suspected coronary artery disease (CAD), the main goal of testing is to exclude the presence of CAD as the cause of symptoms. Selection of the optimal imaging modality for a given patient requires careful consideration of the strengths and weakness of each modality, as well as patient-related factors (e.g., body habitus, conditions that may be contraindications to some forms of testing). Finally, because many modalities can assess other ancillary questions, knowing all the potentially relevant clinical questions for each patient is helpful. Unfortunately, even though a large body of experience exists for most imaging modalities, few direct comparative studies have been conducted.
Figure 10-1 is a decision-making algorithm that can be used in selecting specific tests in patients with suspected CAD. Any such algorithm should serve merely as an initial guide, and clinical judgment, institutional experience, and patient preferences are all important factors that must be considered. A key principle, however, is that the first step in deciding which test to obtain is to determine the pretest probability of flow-limiting (i.e., obstructive) CAD.

Pretest Probability of Disease
Because the diagnostic accuracy of different tests may be affected by the prevalence of disease, the clinician must first determine the pretest probability of obstructive disease. The presence of coronary artery stenosis or even myocardial ischemia does not necessarily imply that the patient’s symptoms have a cardiac origin because an alternative pulmonary, gastrointestinal or musculoskeletal source may also coexist and be the actual cause of the patient’s complaints. This possibility underscores the importance of estimating the pretest probability or, in Bayesian formalism, the prior probability. Only by consideration of a patient’s pretest probability of CAD can a clinician evaluate whether a positive result is more likely to be a true-positive or a false-positive result. In the context of a high pretest likelihood of underlying disease, a negative test result may not be sufficient to exclude underlying disease. On the other end of the spectrum, evaluation of patients with an extremely low pretest probability of disease may result in excessive testing costs, particularly given the high frequency of false-positive results. Thus, to maximize diagnostic accuracy and minimize testing costs, the clinician must restrict testing to patients with an appropriate (e.g., low to intermediate) pretest probability of disease.
Several methods have been developed to estimate a given symptomatic patient’s pretest probability of obstructive CAD based on age, gender, symptoms, and comorbid conditions. Most common among these are the Diamond and Forrester Score and the Duke Clinical Score ( Table 10-1 ). These methods are limited in that they were both developed decades ago (1979 and 1983, respectively) and may no longer reflect contemporary patient populations who are referred for noninvasive imaging.
| Diamond and Forrester Method | Duke Clinical Score | |
|---|---|---|
| Population used to derive score | Combination of (1) symptomatic patients referred for invasive angiography and (2) autopsy studies | Symptomatic patients referred for invasive angiography |
| Prediction factors | Chest pain type Gender Age | Chest pain type Gender Age Smoking Hyperlipidemia Diabetes Previous myocardial infarction (q-waves on electrocardiogram) ST-T wave changes on electrocardiogram |
| Risk score predicts | ≥50% stenosis | ≥75% stenosis |
| Risk categories | Low (<10%) Intermediate (10-90%) High (>90%) | Low (<30%) Intermediate (30-70%) High (>70%) |
Alternatively, many clinicians rely on clinical intuition and experience to compose a pretest probability. Regardless of whether a formal score or an informal estimation is used, formulation of a patient’s pretest probability is an important step in the decision to pursue further testing, in the choice of testing modality, and in the interpretation and application of the test results.
What Are the Available Testing Options?
Nearly every imaging modality used in cardiovascular imaging can be used for evaluating patients who exhibit signs or symptoms suggestive of coronary ischemia. Broadly, these modalities can be divided into anatomic and functional approaches ( Table 10-2 ). Anatomic methods include invasive coronary angiography (ICA) and coronary computed tomography angiography (CTA). In contrast, functional methods employ the concept of stress testing, which refers to a focus on the identification of electrical, perfusion, or contractile abnormalities induced by exercise or pharmacologic stress.
| Physiology and Function | Anatomy |
|---|---|
| Exercise treadmill testing (no imaging) Exercise treadmill testing with imaging
| Coronary artery calcium scoring Coronary computed tomography angiography Invasive coronary angiography |
Anatomic Methods
Invasive Coronary Angiography
To date, quantitative analysis of ICA images has generally been considered the “gold standard” for assessment of CAD. The threshold of 50% or greater stenosis is considered hemodynamically significant in the left main coronary artery, and the threshold of 70% or greater stenosis is considered hemodynamically significant in all other vessels. More recently, investigators have recognized that not all lesions that are visually estimated to cause stenosis truly result in hemodynamic significance. Further, some lesions that are lower than these thresholds may indeed be functionally significant. Consequently, evaluating the hemodynamic significance of CAD is helpful for determining whether potential symptoms are the result of ischemia. Moreover, determining the amount of ischemia can be used to identify which patients are most likely to benefit from coronary revascularization. The gold standard for determining the hemodynamic significance of a particular coronary lesion is to calculate the fractional flow reserve (FFR) by using invasive pressure wires; an FFR of less than 0.8 indicates a hemodynamically significant lesion. However, FFR is an invasive technique that is relatively cumbersome and subsequently is underused. Nevertheless, the landmark Fractional Flow Reserve versus Angiography in Multi-vessel Evaluation (FAME) study showed that, in comparison with visual estimations of stenosis, a strategy of measuring coronary FFR during adenosine infusion with a pressure wire led to fewer coronary revascularizations, better outcomes, and lower cost.
Other invasive intravascular imaging modalities such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) can improve plaque identification and quantification. Although these modalities are invasive and costly, they offer the opportunity to characterize plaque composition and, perhaps, plaque stability more clearly. These features likely have substantial prognostic significance beyond stenosis severity.
Although ICA and newer catheter-based methods afford the most definitive anatomic diagnoses, they require invasive procedures with associated costs and risks. Thus, in most stable patients who do not have a very high pretest likelihood of obstructive CAD, noninvasive testing strategies are preferred.
When to Choose Invasive Coronary Angiography
ICA is the test of choice in unstable patients because of the ability to proceed immediately to therapeutic intervention on ischemia-causing lesions. ICA may also be an appropriate choice in patients with a high (i.e., >90%) pretest probability of obstructive CAD. Even among high-risk patients, however, an initial evaluation of ischemia may be helpful to identify the “culprit lesion” when multiple obstructive lesions are identified. Finally, ICA may rarely be required in patients who cannot exercise and who have contraindications to both vasodilators and dobutamine (e.g., a patient with critical aortic stenosis and asthma who cannot exercise).
Although ICA may be more complicated in patients with large body habitus, adequate results can be obtained. Obesity-related complications are primarily related to vascular access and bleeding, although image quality may also be reduced. The need for iodinated contrast mandates careful consideration of renal function, as well as risk factors for contrast-induced nephropathy.
Coronary Computed Tomography Angiography
Coronary CTA offers a noninvasive approach to visualize the presence of both calcified and noncalcified coronary atherosclerosis and can detect coronary stenosis with high diagnostic accuracy. In particular, CTA has an excellent negative predictive value (~95%) and can thus be used to exclude the presence of obstructive CAD. Conversely, CTA has a lower positive predictive value for identifying the presence of coronary stenosis, in part because of the potential to overestimate the severity of stenosis when extensive coronary artery calcifications (CACs) are present. As is also true for ICA, the presence of anatomic stenosis does not always imply the presence of ischemia. Therefore, patients who are found to have moderate to severe coronary stenosis often require further evaluation to assess the functional significance of disease.
Advantages of CTA include rapid examination time and high accuracy in excluding the presence of plaque or stenosis. Unlike functional techniques, which can identify only flow-limiting lesions, CTA can identify the presence of nonobstructive plaque, which may result in intensification of lifestyle or medical therapies in some subgroups of patients. Another potential advantage of CTA is the ability to provide an alternative explanation for the patient’s symptoms (e.g., pulmonary disease, disease of the aorta).
When to Choose Computed Tomography Angiography
Compared with ICA, CTA avoids the need for an invasive procedure, but it does not eliminate exposure to ionizing radiation or the need for iodinated contrast material. CTA should be considered in symptomatic patients with a low to intermediate pretest likelihood of obstructive disease. Other useful indications for CTA include the following: (1) clarification of inconclusive stress test results; (2) suspected anomalous origin of the coronary arteries; and (3) acute chest pain in selected patients. CTA is not useful in high-risk patients or those with known CAD because the presence of severe CACs may lower the accuracy of the examination or may render the study uninterpretable for evaluating the severity of stenosis. Contraindications to CTA include renal dysfunction, morbid obesity, inability to breath-hold, elevated heart rate, and abnormal heart rhythms.
CTA can identify nonobstructive (i.e., subclinical) lesions, which are unlikely to cause symptoms but are associated with a higher risk of future events. Ultimately, one goal of CTA is to identify high-risk plaques that may be more likely to rupture and lead to future adverse events. Nevertheless, even if such high-risk lesions could be reliably identified, additional research is required to determine the optimal treatment strategy in for such patients.
Physiologic Methods
Physiologic methods of coronary evaluation are based on the identification of signs of myocardial ischemia induced by exercise or pharmacologic stress. The most basic signs are changes on the electrocardiogram (ECG) that indicate myocardial ischemia. Other methods rely on identification of deficits in relative perfusion in areas downstream of coronary stenosis by using a radiotracer or other marker of blood flow. Alternatively, significant ischemia results in regional myocardial systolic dysfunction, which can be directly visualized with functional imaging during stress. The advantage of these methods is that they transcend the weak relationship between the degree of stenosis on anatomic assessment and functional significance. However, most physiologic methods cannot identify lesions that do not cause ischemia but may still have adverse prognostic significance.
Stress Echocardiography
Stress echocardiography protocols are based on the identification of stress-induced wall motion abnormalities following exercise or pharmacologic stress. Advantages of stress echocardiography include widespread availability, portability, lower cost than nuclear and magnetic resonance imaging (MRI) techniques, absence of ionizing radiation, and the ability to evaluate parameters of ventricular and valvular function during the test. Patients with poor acoustic windows (e.g., obese patients and those with obstructive lung disease) may have reduced image quality, which can lower the diagnostic accuracy of the examination. In such cases, image quality may be improved by administration of echocardiographic contrast agents that enhance endocardial delineation. The accuracy of this examination may also be reduced in patients with resting wall motion abnormalities (e.g., prior infarction, severe left ventricular dysfunction, right ventricular pacing, prior cardiac surgery).
When to Choose Stress Echocardiography
Stress echocardiography may be particularly useful in younger patients, in whom radiation exposure may have greater adverse consequences. This technique is also useful in evaluating for exercise-induced dyspnea in patients with valvular heart disease or suspected exercise-induced heart failure with preserved ejection fraction or pulmonary hypertension, although special protocols and expertise are required for such examinations.
Nuclear Methods
Modern nuclear methods include single-photon emission computed tomography (SPECT) and positron emission tomography (PET). These methods are based on the identification of relative differences in myocardial perfusion. Technetium-99m agents are the most commonly used radiotracers and provide excellent image quality. SPECT can be performed in conjunction with exercise or pharmacologic stress. As with most modalities, image quality can be compromised by obesity. This situation can be ameliorated, in part, by performing stress and rest studies on separate days, to allow for increased radiotracer doses. Furthermore, newer-generation SPECT cameras can improve image quality in obese patients.
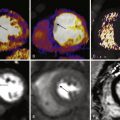
The primary limitation of SPECT methods is the prevalence of artifacts from breast or diaphragmatic attenuation or excess hepatic or gastrointestinal tracer uptake. These artifacts can be reduced by the application of attenuation correction techniques. Less commonly, underestimation of the extent of coronary disease can result from a global or balanced reduction in myocardial perfusion, typically in patients with significant left main or three-vessel coronary disease ( Fig. 10-2 ).