Chapter 137 Rheumatologic conditions in children are myriad in presentation, with overlapping imaging features that may sometimes superficially mimic infection. Rheumatologic conditions in children do not follow the typical course and presentation compared with their counterparts in adults. As a consequence, the International League of Associations for Rheumatology (ILAR) formulated a revised nomenclature to describe the various pediatric rheumatologic conditions (Box 137-1).1 In this chapter, we discuss imaging of pediatric arthritis from the point of view of noninfectious synovial proliferation using the ILAR classification, including juvenile idiopathic arthritis (JIA) and its differentials: hemophilic arthropathy, lipoma arborescens, synovial chondromatosis, pigmented villonodular synovitis, and reactive synovitis (Box 137-2). Subtypes of JIA, enthesitis-related arthritis and psoriatic arthritis, will be discussed in their own subsections because of their unique presentation and imaging findings. JIA, which occurs worldwide, is the most frequent cause of chronic musculoskeletal pain in youths and the most common chronic musculoskeletal disease of childhood.2–4 It is a nonmigratory, chronic, monoarticular or polyarticular arthropathy of childhood.2 The diagnostic criteria for JIA include disease onset before the age of 16 years, the presence of arthritis in one or more joints for at least 6 weeks, onset type defined by type of disease in the first 6 months of diagnosis (Box 137-1), and exclusion of other forms of juvenile arthritis.1 JIA may be associated with systemic manifestations that include fever, erythematous rashes, nodules, leukocytosis, and, less commonly, iridocyclitis, pleuritis, pericarditis, anemia, fatigue, and growth failure.5 At the time of presentation, other causes of inflammation should be excluded. JIA differs from the adult type of rheumatoid arthritis because of the age of presentation, its preference for large joints, its tendency to generate joint contractures and muscle wasting, and its association with extraarticular clinical manifestations.6 A new internationally accepted classification system was established in 19951,7 and revised in 20011 (Box 137-1). The previously used terms “juvenile chronic arthritis” and “juvenile rheumatoid arthritis” were incorporated under the term JIA. The clinical and laboratory tests that are currently available for assessment of JIA are poor for characterization of early inflammatory, hypoxic, and vascular changes, which are the primary physiologic events involved in the disease.8 Hence because the clinical and laboratory diagnosis of early joint changes in JIA is suboptimal,9,10 imaging becomes an ideal noninvasive method for early diagnosis and outcome measure during follow-up of joint changes in persons with this disease. The incidence and prevalence of JIA, respectively, is between 5-18 and 30-150 per 100,000 children younger than 16 years in Europe and North America.11 Twice as many girls as boys have JIA.12 Although few data are available on geographic or racial groups of persons with JIA, studies suggest that in the United States, proportionately fewer African American than white children have JIA.13 The onset of JIA before the age of 6 months is distinctly unusual; nevertheless, the age at onset is often quite young, with the highest frequency occurring between 1 and 3 years.14 Radiographic changes are seen most frequently in patients with JIA who have a polyarticular course.15,16 Large joints are most commonly affected in persons with this disease. The knee is the most frequently affected joint, followed by the ankle. Occasionally, changes may develop in the cervical spine or temporomandibular joint.17 It has been suggested that patients with JIA who have polyarthritis and wrist disease are at high risk of experiencing radiographic progression.18 The wrist is the most vulnerable site for early radiographic changes in patients with JIA.16,19 Although the etiology of JIA is unknown, some investigators believe that it is multifactorial given the heterogeneity of presentations and course of the disease.20 JIA is characterized by an acute synovitis that leads to synovial proliferation and formation of a highly cellular pannus.21 The pannus erodes the adjacent articular cartilage and subchondral bone, leading to centripetal articular destruction; that is, the articular damage starts at the periphery of the joint and progresses toward its center. Inflammatory changes also can involve tendon sheaths and bursa and can give rise to periostitis. With prolonged inflammation, more extensive joint changes including cartilage destruction, bone erosions, and joint malalignment often are present. Despite the fact that JIA is usually transient and self-limited, without active synovitis in adulthood, up to 10% of children become severely disabled in adulthood. Despite therapy, 28% to 54% of children have progressive disease and experience cartilage or bone erosions, with a median onset of radiographic findings between 2.2 to 5.4 years after the initial disease presentation.22 The disease process leads to joint instability, subluxation, and ankylosis.23,24 Disturbance of joint growth can be consequent to the disease itself and/or to the treatment.17 Imaging often plays a key role in establishing the presence, severity, and extent of joint disease, and it can also help monitor for disease complications, exclude other diagnoses, and assess treatment response. Imaging can provide early diagnosis and visualization of inflammatory abnormalities, including synovitis and osteochondral damage.25–27 Radiographs are the standard imaging tools for the diagnosis of JIA; however, they have low sensitivity (50%) and moderate specificity (85%) for detection of cartilage destruction.8 Both magnetic resonance imaging (MRI) and ultrasound can detect synovial hypertrophy, cartilage erosion and joint effusion in peripheral joints, and clinically meaningful response to treatment in children with JIA. Ultrasound is less sensitive than MRI for assessment of both soft tissue findings (sensitivity, 62%) and superficial cartilage loss (sensitivity, 60%).8 Overall, MRI is the imaging modality of choice for evaluation of joints in children with JIA. However, ultrasound can be an excellent initial imaging tool for evaluation of young children who otherwise would require sedation for MRI.8 Conventional radiography is not effective in the evaluation of soft tissue abnormalities, which are precursors of cartilage degeneration in persons with JIA.26 Moreover, available radiographic scoring systems for assessment of JIA have poor internal consistency and poor criterion and construct validity because they do not take into consideration patients’ sex and age.28 Despite the aforementioned limitations and the strong evidence for low sensitivity (50%) and moderate specificity (85%) in the detection of cartilage destruction,8,28 in many centers this technique remains the standard practice for imaging evaluation of disease progression in persons with JIA, with an expanding role for ultrasound and MRI.29 A variety of radiographic features can be encountered with joint disease. Specific joint findings will depend on the underlying abnormality, the chronicity of the disease, and the response to therapy. A systematic approach to the imaging interpretation of any joint is highly recommended. One popular approach is the “ABCDS” of joint disease, featuring assessment of joint Alignment, Bone density and other bone changes, Cartilage loss, Distribution of joint disease (whether monoarticular, oligoarticular, or polyarticular) and Soft tissue abnormalities (Box 137-3). The earliest abnormalities include soft tissue swelling, osteopenia, and effusion. Periosteal reaction occasionally may be seen. Typically, the osteopenia is initially periarticular (Fig. 137-1), becoming more diffuse with time. Osteopenia may be subtle and better recognized by comparison with the contralateral extremity (if it is unaffected). With long-standing disease, uniform bone loss may occur with a thin cortex. Uncommonly, a linear subphyseal demineralization can be observed, but this finding is nonspecific and can also be seen in persons with other conditions such as leukemia.30 Figure 137-1 A 15-year-old girl with polyarticular juvenile idiopathic arthritis. Joint effusions are encountered commonly and can be seen in inflammatory or noninflammatory joint disease. A sign of knee effusions is fullness in the suprapatellar region, which is best seen on the lateral view. In the elbow, knee, and ankle, adjacent fat lines and fat pads are displaced. Periosteal reaction, when present, is commonly seen in the phalanges, metacarpals, and metatarsals but also can occur in the long bones. Joint space narrowing may be caused by cartilage loss (Fig. 137-1). In persons with JIA, the joint space narrowing is usually uniform. In some patients with rheumatoid factor positive polyarthritis or systemic arthritis, early erosive disease can occur (Fig. 137-2). Figure 137-2 A patient with systemic juvenile idiopathic arthritis after having multiple hip infections. Bone erosions are typically located at joint margins in the bare areas but also may occur at tendinous insertions. Bone erosions also can be seen in persons with septic arthritis or hemophilic arthritis related to the inflammatory reaction caused by intraosseous hemorrhage. Large erosions can be seen in the camptodactyly arthropathy coxa vara pericarditis syndrome31 (e-Fig. 137-3). Deformity of the fingers, whether with boutonniere (proximal interphalangeal [PIP] flexion with distal interphalangeal [DID] extension) or swan neck (PIP extension with DIP flexion) deformity, can be seen in a variety of disorders, including JIA (Fig. 137-4), camptodactyly arthropathy coxa vara pericarditis syndrome, or systemic lupus erythematosus. Enlarged or irregular epiphyseal ossification centers can be seen in persons with hemophilia, JIA, and tuberculous arthritis. Atlantoaxial subluxation or cervical vertebrae pseudosubluxation and ankylosis (e-Fig. 137-5) may be noted in persons with JIA, the arthropathy of Down syndrome, dysostosis multiplex, and systemic lupus erythematosus. Figure 137-4 Radiographic findings of advanced juvenile idiopathic arthritis in the peripheral skeleton in different patients. e-Figure 137-5 A child with polyarticular juvenile idiopathic arthritis. Changes in bone growth and maturation with changes in the normal size of ossification centers and alteration of normal bone modeling can be seen in persons with JIA and in persons with infections and hemophilia. Enlargement of ossification centers and epiphyses (Fig. 137-4), contour irregularity, trabecular changes, and squaring (typically of the patella) can be seen. Tibiotalar slant (ankle valgus) also can be noted in persons with JIA.32 Late sequelae of JIA include epiphyseal deformity, abnormal angular carpal bones, widening of the intercondylar notch of knees (Fig. 137-4), and premature fusion of the growth plates. Growth disturbances are more frequent if disease onset is early. Joint space narrowing and osseous erosions are usually late manifestations. At the hip, protrusio acetabuli (Fig. 137-4), premature degenerative changes, coxa magna, and coxa valga can be seen. Joint space loss can progress to ankylosis, particularly in the apophyseal joints of the cervical spine (Fig. 137-5) and wrist. Rarely, ankylosis also can be seen in larger joints, including the hips. Subluxation of the joints, especially at the wrist, may be evident. Growth disturbance of the temporomandibular joint may lead to micrognathia and temporomandibular disk abnormality.32 MRI is an optimal tool for evaluation of both soft tissues and osteochondral abnormalities with superb tissue contrast.33,34 Contrast-enhanced MRI is extremely sensitive for detecting active disease and for early detection of cartilage loss, bone erosions, and synovial hypertrophy in children and adolescents.34 MRI can define vascular anatomy, often without the need for intravenous contrast.35 Higher cost, limited availability, and more frequent need for sedation in younger patients have limited its more widespread use. MRI provides multiplanar evaluation with a combination of available imaging sequences, including T1 and fast spin echo T2-weighted sequences, gradient echo sequences, and postcontrast studies all tailored to the specific clinical problem.36–38 Three-dimensional (3D) isovolumetric sequences also can be obtained, making it possible to reformat images in any desired plane. Cartilaginous structures including the growth plate are well visualized with gradient-recalled echo techniques39 or fat-suppressed fast proton density sequences. Gadolinium-enhanced MRI can help differentiate physeal from unossified epiphyseal cartilage and can visualize normal vessels present within the chondroepiphysis.40 MRI also is helpful in detecting synovial abnormalities within the joint41 and can be used to assess for changes in the synovium with therapy.42 Additionally, MRI can be used to demonstrate muscle pathology, typically demonstrating nonuniform increased signal intensity on T2-weighted images and normal signal on T1-weighted sequences. These findings are not specific but may help in the selection of a biopsy site. Without use of contrast material, proliferating synovium on MRI appears as soft tissue thickening with an intermediate T1- and T2-weighted signal (Fig. 137-6). It may have slightly higher signal intensity than adjacent fluid on unenhanced T1-weighted images. Pannus appears as a thickened intermediate to dark signal intensity on T2-weighted images and is best seen when outlined by bright signal joint fluid. Its variable signal intensity reflects the relative amount of fibrous tissue and hemosiderin. Intravenous administration of gadolinium-based contrast agents improves visualization of thickened synovium, especially with use of fat-suppression techniques. Proliferating synovium appears as enhancing linear, villous, or nodular tissue. Images should be obtained immediately after contrast injection because diffusion of contrast material from the synovium into joint fluid occurs over time. Hypervascular inflamed pannus enhances significantly (see Fig. 137-2, E), whereas fibrous inactive pannus shows much less enhancement. Subchondral cysts and bone erosions (see Fig. 137-2) appear as low signal areas on T1-weighted sequences,36 with overlying articular and epiphyseal cartilage loss better delineated on fluid-sensitive sequences. Meniscal hypoplasia can be seen in some cases of JIA (e-Fig. 137-7).34 Figure 137-6 A 14-year-old girl with juvenile idiopathic arthritis and symptomatic tenosynovitis. e-Figure 137-7 A 5-year-old patient with juvenile idiopathic arthritis. Quantitative techniques have been developed for synovial volume.43,44 MRI is more sensitive than clinical evaluation in detecting some specific joint involvement, including the temporomandibular joint, which often demonstrates inflammatory change in the absence of clinical symptoms.45 With prolonged synovial inflammation, well-defined intraarticular nodules termed “rice bodies” (Fig. 137-8) may be present. Rice bodies likely arise from detached fragments of hypertrophied synovial villi. On MRI, rice bodies have dark signal on T2-weighted images because of their fibrous tissue composition and are associated with joint effusion, synovial hypertrophy, and synovial enhancement after gadolinium administration.46 Bone marrow edema is represented by areas of low T1-weighted and high T2-weighted signal intensity (e-Fig. 137-9) and should be differentiated from normal marrow speckling seen on fluid-sensitive sequences, most frequently in the ankle and foot. Figure 137-8 Rice bodies. e-Figure 137-9 An 11-year-old girl with juvenile idiopathic arthritis presenting with a swollen left ankle and mid foot. Recently, studies in adults47–49 have shown an association between presence of subchondral bone marrow edema in persons with inflammatory arthritis and radiographic erosive progression. Although MRI has being extensively investigated for use in persons with JIA,8,50 standardized measures for data acquisition and interpretation are not currently available51; hence this technique is underutilized both in clinical practice and in research. In addition, the imaging evaluation of growing joints is challenging because thinning of articular cartilage can be either a physiologic or a pathologic process. As a result, early subchondral abnormalities can be masked in joints of young children on MRI because of the greater thickness of epiphyseal cartilage in these joints, which makes evaluation less accurate.8 Very few MRI scales have been designed to specifically assess morphologic changes with JIA.52,53 Novel MRI Techniques: A number of novel MRI techniques are under evaluation for improved assessment of synovial, cartilaginous, or osseous abnormalities (Table 137-1). These MRI techniques include diffusion-weighted imaging (DWI) and perfusion imaging, delayed gadolinium-enhanced cartilage imaging, and T2 quantification. DWI evaluates the translational movement (Brownian motion) of water molecules that occurs in all tissues, including synovium and cartilage. Alteration of normal diffusion can occur in diseases including infection, inflammation, and infarction.54 Diffusion tensor imaging (DTI) is a variant of DWI and has been used to study the structure of ordered biological tissue.55 DTI-derived metrics correlate with inflammatory cytokines and adhesion molecules and holds potential to delineate synovial inflammation; however, it is not superior to conventional MRI in the detection and assessment of therapeutic response.56 Despite the contrast-free characteristics of DWI and the possibility of using 3D steady-state sequences to enable imaging of short-T2 species with high signal-to-noise ratio, the use of DTI to assess cartilage in knee joints is limited because of the short T2 relaxation time of cartilage (30 to 70 msec).57 Table 137-1 Matching of Magnetic Resonance Imaging Sequences to Tissue of Interest Modified from Borrero CG, Mountz JM, Mountz JD. Emerging MRI methods in rheumatoid arthritis. Nat Rev Rheumatol. 2011;7:85-95. Contrast-enhanced perfusion MRI assesses blood flow using intravenously administered paramagnetic contrast agents and may be helpful in characterizing ischemic or hyperemic areas. Potential uses of this technique include recognition of epiphyseal ischemia and quantification and monitoring of synovial inflammation.58 The rate of synovial enhancement depends in part on tissue vascularization and capillary permeability, both of which are highly correlated with synovial inflammation.59,60 Rapid enhancement suggests active synovial inflammation, whereas gradual delayed enhancement suggests subacute/chronic synovial inflammation. The use of pharmacokinetic modeling for quantitative mapping of the perisynovial tissue61,62 can add information to subjective synovial assessment. In this physiologically based model, signal enhancement time courses are described by a plasma compartment and the extracellular extravascular space. Pharmacokinetic parameters that are representative of tissue signal enhancement have been shown to decrease in children with arthritis under treatment over time and provide specific information on synovial inflammation.62 Because cartilage is one of the earliest sites of damage in persons with JIA, it is an important area to be evaluated with MRI. Cartilage has bright signal on both fast spin echo and fat-suppressed proton density sequences; hyaline cartilage has the highest intensity40 and can be differentiated from epiphyseal and physeal cartilage. Articular cartilage should be assessed for areas of altered signal, thinning, erosions, or deep cartilage loss that may extend to the subchondral bone. The development of a variety of fast imaging methods with increased signal-to-noise ratio provide greater cartilage-synovial fluid contrast and have improved the MRI evaluation of cartilage morphology. Fat-suppressed 3D spoiled gradient-recalled echo imaging provides excellent contrast because cartilage has a bright signal compared with adjacent structures, but it is limited in differentiating epiphyseal, articular, and physeal cartilage. Other valuable sequences in cartilage assessment include driven equilibrium Fourier transform, dual-echo steady-state imaging, Dixon water-fat separation technique, and steady-state free precession.35 Delayed gadolinium-enhanced MR cartilage imaging is a sensitive technique for assessing cartilage proteoglycan content using the negative charge of the intravenously administered paramagnetic MR contrast agent.63 The contrast agent distributes into cartilage inversely to the fixed charge density of negatively charged glycosaminoglycan (GAG). T1 relaxation time in the presence of gadolinium agent is approximately linearly related to GAG content. Delayed gadolinium-enhanced MR cartilage imaging may be used to assess early cartilage injury with depletion of GAG before anatomic changes are visible by conventional cartilage sequences. Cartilage assessment can also be provided by mapping T2 relaxation time measurements. These measurements may help characterize the structural integrity of the cartilaginous tissue and quantitatively assess the degree of cartilaginous hydration and collagen orientation.64 Typically an overall decrease in T2 relaxation from the cartilage surface to the deeper layers occurs.65,66 In persons with JIA, increased cartilage T2 relaxation time is thought to be an early marker of disease progression in JIA, because it can identify microstructural changes before damage becomes visible.67 In a longitudinal study evaluating patients with JIA from 3-month to 2-year follow-up, the clinical assessments improved, whereas T2 maps showed increased T2 values.68 This increase likely represents progressive microstructural changes, even though clinical symptoms improved with treatment. Another alternative MRI method is Na23 MRI,69,70 which identifies areas of proteoglycan depletion through bonding of the positively charged sodium with negatively charged GAG molecules.71 The major limitation of this technique is the low overall sodium content in cartilage, which limits the signal-to-noise ratio. MRI protocols using higher strength field MRI scanners (7 tesla) have been devised to try to overcome this technical challenge.72–74 With the improved signal-to-noise ratio of higher strength field MRI scanners and dedicated transmit-receive radiofrequency coils, signal-to-noise ratio can be increased while specific absorption rate is reduced, which should facilitate imaging of small joints.75,76 Recent advances in ultrasonography, including better transducers and more pediatric musculoskeletal experience, have stimulated increased use of ultrasound in the assessment of pediatric joint disease. Ultrasound is ideal for assessing the pediatric musculoskeletal system largely because of its ability to visualize intraarticular structures such as cartilage and thickened synovium without the need for radiation. Ultrasound is very sensitive in detecting joint effusion, particularly in the hip (see Fig. 137-2, C) and shoulder, where radiographs are insensitive.77 Intraarticular masses also may be detected with ultrasound, although their appearance is often nonspecific. Tendons and ligaments also can be assessed with higher frequency transducers.78 Fluid within the synovial sheath appears as an anechoic halo surrounding the tendon (Fig. 137-10), whereas synovial thickening appears as a hypoechoic thickening around the tendon. Vascular anatomy can be assessed by combining sonography with Doppler. Synovial hyperemia leads to increased Doppler signal.79 Power Doppler has been shown to detect residual disease activity more sensitively than clinical examination and/or MRI both in active disease and when JIA is in remission80,81 and could be used to predict short-term relapse in patients with JIA who appear to be in remission clinically.82 Figure 137-10 A 10-year-old girl with polyarticular juvenile idiopathic arthritis. Sonography can also be used to assess other periarticular soft tissue abnormalities, including popliteal cysts (e-Fig. 137-11) or other soft tissue masses, and to guide aspiration or injection of joints.83 e-Figure 137-11 A Baker’s cyst. Disadvantages of ultrasound include lack of standardization of ultrasound techniques for assessment of growing joints and normative literature data, lack of capability to visualize the central aspect of some joints, and difficulty in assessing some joints, such as the temporomandibular joints (Fig. 137-12).45,84 Data on the diagnostic accuracy of ultrasound in children with JIA are limited. Assessment of joint effusion, synovial hypertrophy, and cartilage erosions by ultrasound can provide information about the severity of the disease. Figure 137-12 A 12-year-old girl with longstanding juvenile idiopathic arthritis and symptomatic and asymmetrically involved temporomandibular (TMJ) joints clinically. Erosions and focal or diffuse thinning of the articular cartilage also can be detected, but only peripherally in the joint. Color Doppler ultrasound enables the detection of perisynovial hyperemia. Studies in children85,86 have demonstrated the ability of color and power Doppler sonography, with or without intravenous injection of contrast agents, to estimate synovial activity in JIA. Resistive indices and fraction of color pixels may be used as quantitative measurements of the blood flow.87,88 Contrast-enhanced sonography holds potential for detection of active synovial inflammatory disease in persons with subclinical JIA and may help guide early treatment.85 Very limited information is available about the diagnostic performance of ultrasound compared with MRI or clinical examination (in knees, sensitivity for joint effusion is 62%,89 ranging between 60% and 90% for clinically active joints and approximately 70% for clinically inactive joints90–92; for superficial cartilage destruction, overall sensitivity is 60%).89 Ultrasound-determined synovial thickness of the knee seems to correlate with clinical and laboratory (sedimentation rate and C-reactive protein levels) disease activity scores and with biomarkers of disease activity.92 In ankles, however, very poor agreement was observed comparing clinical and ultrasound scores.93 A recent systematic review on the best evidence for treatment of JIA94 showed that nonsteroidal antiinflammatory drugs are effective only for a minority of patients, mainly those with oligoarthritis. Intraarticular corticosteroid injections are very effective for persons with oligoarthritis. Methotrexate is effective for the treatment of persons with extended oligoarthritis and polyarthritis and less effective for persons with systemic arthritis. Sulfasalazine and leflunomide may be alternatives to methotrexate. Antitumor necrosis factor medications are highly effective for polyarticular JIA that is not responsive to methotrexate but are less effective in persons with systemic arthritis. Therefore despite many advances in the treatment of persons with JIA, evidence is still lacking for treatment of several disease subtypes. With regard to the use of intraarticular corticosteroids, studies have shown that as many as 70% of patients with oligoarthritis do not have reactivation of disease in the injected joint for at least 1 year, and 40% do not have reactivation for more than 2 years.95–97 Radiographic and MRI studies have shown a marked decrease in synovial volume after injection without deleterious effects on the cartilage.98 Radiography: Radiography is able to demonstrate epiphyseal overgrowth and osteopenia after the injection of intraarticular triamcinolone hexacetonide.99 Carpal length, defined as the radiometacarpal length plotted against the length of the second metacarpal bone on a chart with normal growth carpal scores, as described by Poznanski et al.,100 is another parameter that has been used in follow-up with an interval increase in carpal length (a positive change) indicating improvement. Sonography: Eich et al.99 used ultrasound to determine the presence of effusion, pannus, popliteal cysts, and lymphadenopathy in 10 children with JIA affecting 15 joints (11 knee and 4 hip joints) before and after intraarticular therapy and concluded that ultrasound was as sensitive as MRI in demonstrating joint effusion and/or pannus but that differentiation between the two was difficult, particularly in the hip joint. Sureda et al.90 reported that 2 out of 16 patients (12.5%) had marked clinical improvement with a corresponding decrease in cartilage thickness (insufficient evidence). Magnetic Resonance Imaging: Although CT is able to demonstrate joint space narrowing, erosions, and condylar flattening,101 MRI is currently the modality of choice to document changes before and after therapy. MRI can be used to monitor cartilage and bone erosions, effusion, pannus, and synovial volumes.39 In studies of children with arthritis who received intraarticular steroid injections, MRI has shown that intraarticular steroid therapy has a long-lasting beneficial effect, with suppression of synovial inflammation and reversion of pannus formation.98,99,103 Quantitative dynamic contrast-enhanced MRI based on pharmacokinetic modeling can be used to evaluate disease activity in the knee. A study of pharmacokinetic parameters and synovial volumes showed significant decreases at 12 months after intraarticular steroid therapy; however, improvement in synovial volume appeared to lag behind dynamic parameters, reflecting delay or subclinical synovitis.62 Of all the imaging modalities, MRI has been shown to be the most sensitive modality in the assessment of temporomandibular joint arthritis in children and has been used as a reference standard measure for comparison of clinical examination and ultrasound in clinical studies.45
Arthritis and Differential Inflammatory Joint Disorders
Juvenile Idiopathic Arthritis
Epidemiology
Pathophysiology
Imaging
Radiography

A frontal radiograph of the hands and wrists (A) shows periarticular osteopenia, erosive changes in the scaphoid, capitates, hamate, and triquetrum bilaterally, and joint space narrowing at the radiocarpal and carpal-metacarpal joints of the second and third digits. Radiographs of the feet (B and C) show flattening and subchondral sclerosis of the metatarsal head of the first right toe, suggesting avascular necrosis (arrow, B), and a right calcaneocuboid joint space narrowing (arrow, C) that was believed to be related to underlying inflammatory changes. A frontal radiograph of the elbows (D) demonstrates overgrowth of epiphyses (medial humeral epicondyles and radial heads) bilaterally.

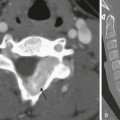
At the age of 4 years, only slight irregularity of the contour of the proximal femoral epiphyses is noted on a frontal radiograph (A) representing a variation of normal, with preserved joint spaces. At the age of 11 years, extensive erosive changes are seen in the femoral heads and acetabula with further joint space loss at the hips bilaterally, as seen on radiographs (B). Interval increased sclerosis is noted along the acetabular roof bilaterally. Nonspecific periosteal reaction is seen along the medial aspect of the femoral necks bilaterally (arrows). Sclerotic lines are shown along the iliac wings (arrowheads) compatible with previous bisphosphonate therapy. Gray-scale ultrasound images (C) obtained at the age of 11 years, 1 month before the corresponding magnetic resonance (MR) imaging scan, demonstrate moderate left hip joint effusion and mild right joint effusion. On an unenhanced multiplanar gradient recalled acquisition MR image (D), a contrast-enhanced coronal T1-weighted spectral presaturation inversion recovery MR image (E), and a multiplanar gradient recalled acquisition MR image (F) at the age of 11 years, markedly thickened, lobulated, heterogeneously enhancing synovium is seen in both hip joints. Enhancing signal abnormality, subchondral cysts, and surface irregularity are seen along the superior compartment of the hip joints. Marked reduction in the hip joint spaces is seen bilaterally, with flattening of femoral heads. The findings are likely to represent severe progression of inflammatory arthritis with diffuse pannus formation. Bilateral secondary avascular necrosis of the femoral heads is noted.

A, Patient 1: Deformity of the digits as a result of erosive changes is seen at the phalangeal heads and bases (white arrows) and metacarpal and carpal bones (black arrow), along with joint space narrowing, carpal ankylosis (arrowhead), and array subluxation. B, Patient 2: A radiograph shows joint space narrowing, advanced maturation, and epiphyseal overgrowth (shown in the distal left tibia and fibula in comparison with normal-appearing right counterparts). C, Patient 3: Widening of intercondylar notch of the knee. D, Patient 4: Protrusio acetabuli.

A lateral cervical radiograph demonstrates diffuse ankylosis of the posterior elements of C3-T1.
Magnetic Resonance Imaging

Sagittal T1-weighted (A) and fat-saturated T2-weighted (B) magnetic resonance (MR) images of the right ankle show synovial overgrowth within the tibiotalar (arrows) and intertarsal joints. Extensive erosive changes and bone marrow edema are noted at the tibiotalar joint (A and B). A small amount of fluid is seen within the synovial sheaths of the tendons of the medial aspect of the right foot, including the tibialis posterior (long arrow) and flexor hallucis longus (short arrow) on axial T2-weighted MR images, suggesting tenosynovitis (C). Longitudinal gray-scale (D) and color Doppler (E) ultrasound scans demonstrate a small amount of fluid noted within the synovial sheaths of the tendons of the medial aspect of the right foot (D) with associated local hyperemia (E).

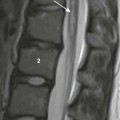
A coronal T1-weighted magnetic resonance image shows meniscal hypoplasia (arrow) in the medial meniscus of the left knee.

A 3-year-old boy had persistent knee pain 1 month after sustaining a trauma. The lateral radiograph view (A) shows moderately large joint effusion distending the suprapatellar bursa. Intraarticular elongated bodies are seen on axial multiplanar gradient-recalled acquisition (B) and T2-weighted fat-saturated (C) and contrast-enhanced T1-weighted fat-saturated (D) magnetic resonance images; these bodies, which extend into the recesses of the joint space and layer in dependent portions, are so-called “rice bodies.” Marked synovial enhancement also is noted. These findings suggest an underlying inflammatory arthritic process without associated joint destruction.

The radiograph (A) and magnetic resonance imaging (MRI) examination (B and C) were obtained 1 week apart. Unenhanced axial inversion-recovery (B) and postcontrast axial T1-weighted spin echo images (C) of the left foot obtained with fat saturation are shown. Soft tissue swelling is present along the left ankle (A) overlying the medial malleolus (arrows). Also present are marked osteopenia of the ankle, hindfoot, and midfoot and talonavicular, calcaneocuboid, and navicular-cuneiform joint space narrowing (A). Assessment of joint space narrowing is best performed with radiography. Although no erosions are identified on MRI, bone marrow edema is noted predominantly within the anterior aspect of the talus, navicular, and cuneiforms. Increased signal intensity on inversion recovery images (B) and contrast enhancement (C) is also noted in the soft tissues surrounding the involved bones, but the metatarsophalangeal and interphalangeal joints are unremarkable (not shown).
Tissue
Measurement
MRI Sequence/Technique
Synovium
Fluid
T2-weighted fast spin echo MRI
Rate of transfer of contrast between plasma and extravascular extracellular space
Dynamic contrast-enhanced MRI
Restricted water motion (inflammation proxy)
Diffusion tensor imaging
Cartilage
Cartilage hydration and collagen orientation
T2-mapping MRI
Glycosaminoglycan content
Delayed gadolinium-enhanced MRI
Proteoglycan depletion
23Na MRI
Proteoglycan content
T1 rho MRI
Bone
Erosions
T1-weighted MRI
Bone marrow edema
T2-weighted fast spin echo or short tau inversion recovery MRI
Ultrasonography

The longitudinal gray-scale ultrasound scan along the medial aspect of the patient’s left ankle shows an increased amount of fluid within the posterior tibialis tendon (A). Corresponding transverse (B) sonograms demonstrate tenosynovitis involving the posterior tibialis (PT), flexor digitorum longus (FDL), and flexor hallucis longus (FHL) tendons of this ankle.

A 4-year-old girl has an asymptomatic lump in the popliteal fossa of the right knee. The lateral radiograph (A) is unremarkable, but the transverse sonogram (B) through the popliteal fossa demonstrates a large cyst with a tail (arrow) extending toward the knee joint.

A coronal T1 image (A), a contrast-enhanced coronal T1 spectral presaturation inversion recovery (SPIR) image (B), a contrast-enhanced T1 SPIR image (C, right TMJ), a sagittal proton density image (D, left TMJ), and a sagittal PD image (E, right TMJ) show moderate right acute TMJ synovitis and mild left acute synovitis. Established chronic arthropathic changes are seen in the left TMJ joint with definite erosive changes. Incipient evidence of chronic arthropathy is noted in the right TMJ. Note a hypoplastic left mandibular ramus in A and B.
Treatment and Follow-Up
Imaging in the Assessment of Response to Therapy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree