Chapter 104 Overview: First described at autopsy by von Rokitansky in 1861,1 the term superior mesenteric artery (SMA) syndrome refers to obstruction of the third portion of the duodenum at its crossing between the aorta and the SMA. It also is known by other terms, including cast syndrome and Wilkie syndrome after David Percival Wilkie, who termed it “chronic duodenal ileus” in his description of 75 patients in 1927.2 The overall prevalence is cited to be between 0.013% and 0.3% of patients undergoing upper gastrointestinal (UGI) examinations.3–6 Etiology: The normal angle between the aorta and the SMA is between 25 and 60 degrees, and the normal aorto-SMA distance is 10 to 28 mm,3 parameters that have been found to be significantly correlated to adult body mass index, particularly in females.7 The etiology of SMA syndrome has been ascribed to an abnormally low angle between the aorta and the SMA, bringing them in close apposition at the crossing of the third portion of the duodenum. The abnormally low angle and decreased distance between the aorta and the SMA is thought to occur because of an abnormally low origin of the SMA or an abnormally high position of the duodenojejunal junction at the ligament of Treitz, so that the third portion of the duodenum is located nearer to the origin of the SMA.1,7,8 Although asthenic individuals, particularly after acute weight loss, are most susceptible to the development of SMA syndrome, in a series by Biank et al., it was found that this presentation occurred in only approximately 50% of their patients. In their study population, the body mass index ranged between the 3rd and 97th percentile for age, with a mean at the 39th percentile. Premorbid conditions included Nissen fundoplication, cerebral palsy, traumatic brain injury, and posterior spinal fusion.6 Other persons predisposed to SMA syndrome include burn victims and patients with lumbar hyperlordosis or who are in spinal traction or a spinal cast.9 According to one report the syndrome occurred in several members of one family, raising the question of genetic predisposition.10 Clinical Presentation: The clinical syndrome occurs more commonly in females between the ages of 10 and 39 years, although it has been described rarely in neonates.11–13 The most common clinical presentation includes abdominal pain, vomiting, nausea, early satiety, and anorexia6,14; acute presentation may follow one of the aforementioned premorbid conditions. Imaging: Plain radiographs may show gastric dilatation, but the stomach may be decompressed by vomiting or by an enteric tube. Contrast UGI study shows dilatation and partial obstruction of the third portion of the duodenum, terminating in a straight line at the expected location of the crossing of the SMA, with active but poorly effective peristalsis across the point of obstruction (Fig. 104-1, A). A useful maneuver to confirm that the obstruction is a consequence of SMA syndrome is to place the patient in a left side down decubitus position, which tends to relieve SMA obstruction. Figure 104-1 Superior mesenteric artery syndrome. Ultrasound has been used to measure both the angle and the distance between the aorta and the SMA but may have difficulty outlining their relationship to the duodenum unless the latter is fluid-filled. Computed tomography (CT) (Fig. 104-1, B and C) and magnetic resonance imaging (MRI) are able to assess the dilatation of the duodenum, as well as its relationship to the SMA. Treatment: Nonoperative treatment centers on weight gain with high-calorie enteral nutrition either by mouth or transpyloric tube feedings beyond the point of obstruction, or with hyperalimentation; such nonoperative intervention is successful in most patients.6 Medical treatment is most likely to be successful when the syndrome is of acute onset.15 Operative treatment is reserved for patients in whom medical management fails and typically consists of a duodenojejunostomy.16 Some authors advocate obtaining a biopsy specimen of the duodenum and jejunum before undertaking surgery to exclude infection, an infiltrative or neoplastic process, or intestinal pseudoobstruction as a cause of the duodenal dilatation.1 Overview: A duodenal hematoma is an infrequent injury in pediatric patients, occurring in fewer than 3% of children with abdominal injuries, and usually is associated with blunt abdominal trauma, both accidental and nonaccidental. The hematoma often extends beyond the ligament of Treitz into the proximal jejunum as a duodenojejunal hematoma.17 In a series of 33 children with a blunt duodenal injury, nonaccidental trauma was the most common cause of duodenal injury (24%), followed by motor vehicle crashes, handlebar injures, sports injuries, and other forms of direct trauma.18 Etiology: The retroperitoneal duodenum contains a vascular submucosal and subserosal plexus and lies relatively fixed against the rigid spine, thus facilitating injury after blunt abdominal trauma. Bleeding is contained within the submucosa and subserosa and encroaches into the lumen of the duodenum. In children, this injury typically occurs after directed blunt trauma such as a direct punch or kick to the epigastrium, or as a result of handlebar injuries. Motor vehicle injuries such as those involving sudden deceleration forces also can produce a shearing injury and result in an intramural hematoma.17,19 Duodenal hematomas also have been reported in patients with coagulation abnormalities after a relatively minor trauma, after undergoing an endoscopic duodenal biopsy,19–23 or when pancreatitis develops, with the latter postulated to be a result of disruption of the intramural vasculature by pancreatic enzymes.24,25 Clinical Presentation: The clinical presentation includes abdominal pain and vomiting, which typically is bilious. When a duodenal hematoma occurs after blunt trauma, pancreatitis related to an associated pancreatic injury may develop. A luminal obstruction sufficient to produce symptoms may not occur immediately after injury, and the child may not present until several days after the traumatic event. When a duodenal hematoma occurs after significant trauma such as a motor-vehicle accident, the CT examination may show the diagnosis before symptom onset. One should always remember that this injury, in the appropriate clinical setting, should raise the concern for inflicted trauma.17,20,26 Imaging: Plain radiographic films may be the initial modality in a child presenting with epigastric abdominal pain, tenderness, and vomiting. Depending on the degree of obstruction and the severity of vomiting, the radiographs may show distension of the stomach and duodenum, with paucity of distal bowel contents (Fig. 104-2, A). Figure 104-2 Duodenal hematoma. Findings of a UGI examination depend on the ability of some of the contrast material to traverse the obstructed portion of the duodenum. If no or very little contrast material moves through, the luminal border of the distended duodenum may show a lobulated contour. As contrast material passes through the obstruction, the lobulated contour of the filling defect will outline the column of contrast across the duodenum (Fig. 104-2, B). Smaller hematomas may demonstrate more subtle findings, such as some thickening of the duodenal folds.17 Ultrasound (Fig. 104-2, C) typically shows a distended first portion of the duodenum, with a filling defect encroaching upon and obliterating its lumen.27,28 The echogenicity of the hematoma is variable, depending on the status of the clot; the clue to the diagnosis is the fact that the mass follows the course of the duodenum. On Doppler interrogation, the lesion has no flow. CT demonstrates similar findings, with dilatation of the duodenum and a nonenhancing mass that follows the course of the duodenum, obliterating its lumen (Fig. 104-2, D and E). MRI often is not performed in children with duodenal hematomas. If it is performed, it again will show a mass following the course of the duodenum, obliterating its lumen, with signal characteristics consistent with an evolving hematoma that may appear in concentric layers of differing signal intensity.29 Treatment: Patients with duodenal perforation need emergent surgery, but the treatment for a duodenal hematoma generally consists of decompression of the stomach and duodenum via a nasogastric tube and maintenance of hydration and nutrition with total parenteral nutrition until the hematoma resolves. In one series of 27 children with a duodenal injury who were treated conservatively, the mean time to resolution of symptoms ranged between 12 and 16 days.18,30 If complete obstruction is present without improvement, it may be necessary to evacuate the hematoma surgically.18 Overview: Duodenal intussusception may be seen in both antegrade and retrograde directions, typically around a gastrojejunal (GJ) tube. Etiology: Antegrade duodenojejunal intussusception is a relatively common complication of the placement of postpyloric GJ tubes, because the tube acts as a lead point for the intussusception. Children with GJ tubes have a 16% to nearly 50% rate of antegrade intussusception.31,32 It is believed that intussusceptions are more likely in boys, in younger patients, in patients with larger bore tubes and tubes with a distal pigtail that may act as a lead point, and when prokinetic agents are administered.32,33 Intussusception also can occur around a weighted nasojejunal tube.34 Retrograde intussusception, on the other hand, is much less common and is believed to occur as a tube with an inflated terminal balloon, which has migrated distally as a result of peristalsis, is pulled back toward the stomach.35,36 Clinical Presentation: The presentation typically is the onset of vomiting, which is bilious in approximately half the cases31 and may be accompanied by abdominal pain/irritability. However, sometimes these intussusceptions are asymptomatic and are discovered at the time of a routine tube check,37 and therefore they may be transient. Hematemesis or bloody gastric aspirates typically are described in patients with retrograde intussusceptions.35,36 Although it bypasses the duodenum, retrograde intussusception also occurs after gastric surgery, such as a gastrojejunostomy. The intussuscepted jejunum may be gangrenous; obstruction and hematemesis are typical in acute presentations.38 Imaging: Abdominal radiographs and ultrasound are helpful in making the diagnosis (e-Fig. 104-3). Radiographs may reveal gastric outlet obstruction, and in cases of retrograde intussusception, gas within the stomach will demonstrate the distal mass protruding into the gastric antrum. Ultrasound demonstrates the classic appearance of bowel within bowel at the gastric outlet31,35,39 and may show a balloon as a lead point. Particularly in retrograde intussusception, upper GI examination will demonstrate the classic “coiled spring” appearance as the contrast material passes into the intussusception; the amount of contrast material may be small and the findings relatively subtle, and thus a high index of suspicion is helpful in arriving at the correct diagnosis.39 On a CT image, the intussusception will be seen extending along the course of the duodenum, and it is seen particularly well when it is outlined by air or contrast medium.40 e-Figure 104-3 Retrograde duodenogastric intussusception in a 3-year-old boy with a gastrostomy tube who presented with vomiting. Treatment: Introduction of air or saline solution through the tube has been successful in reducing intussusception,31 although antegrade intussusceptions can be treated by replacement of the GJ tube over a wire.33 This complication can be prevented by changing to a smaller bore GJ tube, using a catheter without a pigtail, or shortening the length of GJ tube.41 If the intussusception occurs around a nasojejunal tube, the tube should be removed.37 Retrograde intussusception is usually managed by deflation of the balloon,35 although in some cases of supervening bowel ischemia, surgical resection may be necessary.42 Overview: Ulcer disease can involve the duodenum and, as in the stomach, it is associated with Helicobacter pylori infection, a pathogen that is estimated to colonize or infect approximately half the world’s population.43 The prevalence of ulcer disease is lower in children than in adults, but duodenal ulcers are more common than gastric ulcers in children after the neonatal period. Etiology: Duodenal ulcerations result when the inherent defense mechanisms are overwhelmed, including epithelial cell renewal and regeneration, duodenal bicarbonate production, preservation of mucosal blood flow, and production of prostaglandins.44 The understanding of peptic and duodenal ulcer disease in both adults and children has changed dramatically since the elucidation of the role of H. pylori infection (then known as Campylobacter pyloridis) in its development by Warren and Marshall in 1984,45,46 for which they were awarded the Nobel Prize in 2005. Because H. pylori is trophic for gastric epithelium, duodenal ulcers related to H. pylori are believed to occur at sites of gastric metaplasia47 and thus are related to increased acid secretion and decreased bicarbonate output by the duodenum, resulting in lower luminal pH and precipitating bile acids, which otherwise would exert an inhibitory effect on the growth of H. pylori. Acid hypersecretion additionally promotes the activation of pepsinogen to pepsin, facilitating mucosal ulceration.47 A higher rate of H. pylori infection has been reported in children with duodenal ulcers (62%) than in those with peptic ulcers (20%), although other sources do not always confirm this relationship.48 Transmission of H. pylori is mainly by the oral-oral or fecal-oral route and requires contact with intestinal secretions; light contact, such as kissing, is not believed to be effective.49,50 Colonization with this pathogen has greater prevalence in locations with suboptimal sanitary infrastructure.50 Childhood is the primary period of colonization by H. pylori, with cases often clustering within families as a result of intrafamilial transmission.50 In Western nations the prevalence of H. pylori colonization is decreasing and has been reported in as few as 27% of children from Western European centers, although it is thought that some underreporting may be occurring because the specimens are being cultured from the stomach rather than the duodenum. Clinical Presentation: Children with duodenal ulcer disease may present with epigastric tenderness, pain that wakes the child from sleep at night, hematemesis, melena, anorexia, poor weight gain, and vomiting.48,51 Other manifestations include failure to thrive with growth disturbance and poor weight gain, iron deficiency anemia, and idiopathic thrombocytopenic purpura.50 Imaging: Fluoroscopy and upper GI examinations largely have been supplanted in the diagnosis of duodenal ulcer disease by upper endoscopy, which has become the gold standard for diagnosis, in addition to allowing biopsy and culture of detected abnormalities. When upper GI examination is performed, double contrast is more sensitive in the detection of ulcer disease, although this procedure is much more difficult to perform in younger patients and impossible to perform in infants. Despite the shift to endoscopy, duodenal ulcers should be detected during upper GI examination if they are present. Duodenitis, which can be a result of ulcer disease, is manifested by thickening of the duodenal folds, and this finding has a sensitivity of 45% in detecting duodenal inflammation (e-Fig. 104-4).51 Free peritoneal air may be seen if perforation has occurred (Fig. 104-5, A). Barium is the usual contrast agent, but contrast material that has a high iodine concentration and low osmolality and is water soluble should be used if perforation is a concern. The imaging appearance of duodenal ulcers in children is similar to that in adults. The most common finding in an acute ulcer is contrast within an ulcer crater (Fig. 104-5, B). Radiating folds that represent surrounding mucosal edema also may be seen. When the ulcer is on the nondependent wall of the duodenum, air will fill the ulcer outlined by barium. Chronic duodenal ulcers may lead to a scarred and deformed duodenum, but this appearance is less common in children than in adults. So-called “giant duodenal ulcers” are rare and occur more commonly in association with NSAIDs, in patients with end-stage renal disease, and in patients with Crohn disease.47 Figure 104-5 A duodenal ulcer.
Acquired Abnormalities
Duodenal Obstruction
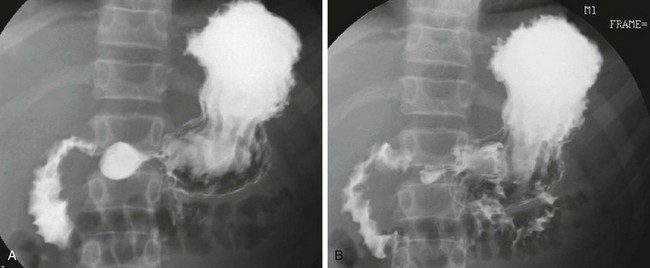
Superior Mesenteric Artery Syndrome
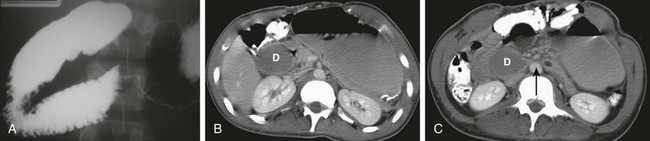
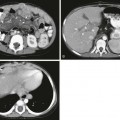
A, A 15-year-old girl with rapid weight loss. An anteroposterior spot image from an upper gastrointestinal examination demonstrates complete obstruction at the third portion of the duodenum. B and C, Two images from a computed tomography scan in a 22-year-old who had sustained a gunshot wound, resulting in paraplegia. The stomach and duodenum (D) are dilated. The arrow points to the compression of the duodenum at the crossing of the superior mesenteric artery, with resolution of dilatation of the duodenal lumen beyond the point of compression.
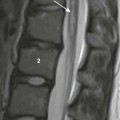
Duodenal Hematoma
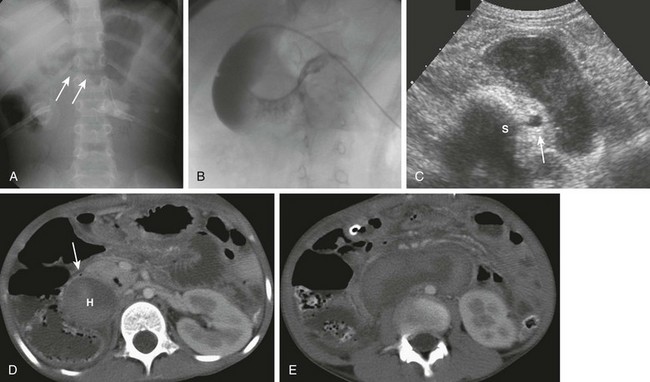
A, An 8-year-old with agenesis of the corpus callosum who had undergone an upper endoscopy with a duodenal biopsy and subsequently developed persistent bilious vomiting. The abdominal radiograph shows a polypoid density (arrows) encroaching upon the gas along the inferior border of the antrum. Gaseous distension of the duodenum is seen with very little gas distally. B, An upper gastrointestinal examination from a 10-year-old boy with cerebral palsy and mental retardation who presented with dehydration and inability to tolerate feedings. Introduction of contrast material into the duodenum during an unsuccessful attempt at gastrojejunal tube placement shows an obstructing duodenal hematoma as a filling defect upon the third and distal second portions of the duodenum. C, An abdominal ultrasound on the same patient shown in part A demonstrates the heterogeneous duodenal hematoma, following the course of the third portion of the duodenum. S, Spine. Arrow points to the aorta. D, A computed tomography (CT) image at the level of the junction of the second and third portions of the duodenum in the same patient in parts A and C shows the large heterogeneous hematoma (H) obliterating the duodenal lumen. The arrow points to the duodenal wall and gas bubble in the residual obliterated lumen. E, A more distal CT section through the third portion of the duodenum (an analogous section to ultrasound in part C) shows again the heterogeneous hematoma, which is obliterating the lumen of the third portion of the duodenum.
Duodenal Intussusception
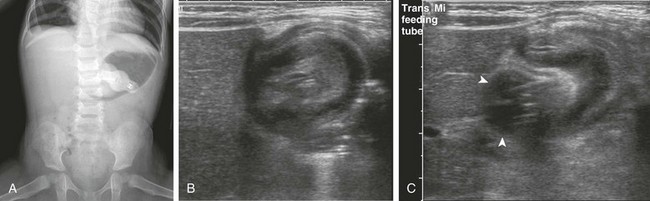
A, An anteroposterior view of the abdomen demonstrates a gastrostomy tube, a dilated air-filled stomach with a masslike lesion at the antrum, and a paucity of distal bowel gas consistent with gastric outlet obstruction. B, Right upper quadrant ultrasound scan demonstrates an intussusception. C, An ultrasound image immediately adjacent to that in part B confirms that the gastrostomy tube balloon (arrowheads) is intimately related to the intussusception.
Duodenal Inflammatory Conditions
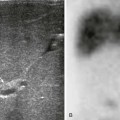
Duodenal Ulcer Disease
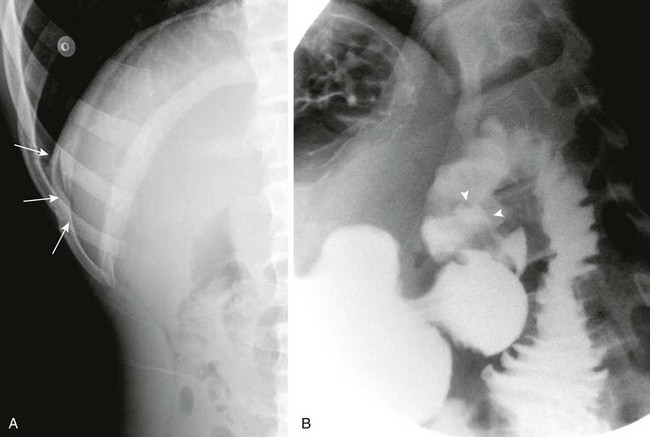
A, A left lateral decubitus radiograph in a boy with a 1-week history of abdominal pain demonstrates pneumoperitoneum lateral to the liver (arrows). B, A spot image of the duodenal bulb from an upper gastrointestinal (UGI) examination with water-soluble contrast material for the same patient shown in part A reveals a collection of contrast in the ulcer crater (arrowheads). (B, Courtesy Dr. Tamar Ben-Ami, Chicago, IL.)