3 BI-RADS For Ultrasound Along with the fourth edition for mammography and the first for breast MRI, the Breast Imaging Reporting and Data System–ultrasound (BI-RADS–US) was published in 2003 by the American College of Radiology (ACR) after 5 years of development and validation. The project was supported in part by grants for protocol design for breast ultrasound research from the United States Public Health Service’s Office on Women’s Health and the National Cancer Institute. Topics that were designated for research included ultrasound for breast cancer screening, differentiation of benign/malignant solid masses, and the possible therapeutic applications of ultrasound, using ultrasound as a therapeutic agent (high-frequency ultrasound) as well as a guide for breast interventions. An international Expert Working Group was assembled by the ACR that included the authors of this text. When the participants convened in Maine in 1998 they immediately recognized the need for a consistent and universally understood terminology to underlie projects using a modality as operator dependent as ultrasound, particularly when the projects involved the identification of criteria to apply in assessing an abnormality’s likelihood of malignancy. Even at the time of the Maine meeting, ultrasound was entrusted only with cyst–solid differentiation. Encouraged by improvements in ultrasound systems and transducers, experienced breast ultrasonologists began to appreciate that if their technique were optimized and the language of interpretation standardized, ultrasound analysis of a mass might move beyond cyst vs. solid, and moreover, that a lexicon might aid in identifying feature patterns of benign solid masses as well as malignant lesions. Groups of characteristics analogous to those in mammography might emerge to allow follow-up for those masses with benign characteristics and biopsy for malignant-appearing or indeterminate lesions. The measure of success of a lexicon would be its potential to increase the specificity of solid mass assessments. Shortly after the Maine meeting, a subcommittee of the ACR’s BIRADS Committee, then co-headed by Carl D’Orsi and Daniel B. Kopans, was formed to develop a BI-RADS for ultrasound. Ellen B. Mendelson chaired the subcommittee, which included Janet Baum, Wendie A. Berg, Christopher R. B. Merritt, and Eva Rubin. BI-RADS–US was modeled on the system used for mammography, in widespread clinical use in the United States. BI-RADS offered a method for quality assurance and outcome analysis, and its final assessment phrases had been incorporated into the Mammography Quality Standards Act of 1992. Anticipating breast imaging becoming increasingly multimodal in its approach, wherever the anatomic descriptors and feature categories of BI-RADS for mammography were applicable to ultrasound, we incorporated them into the ultrasound BI-RADS. The scheme was completed by adding feature categories and descriptors unique to ultrasound. The proposed lexicon went through several versions, and the consensus document was presented and tested by breast imagers at several meetings, the first being the Society of Breast Imaging biennial meeting in San Diego, California in 2001. Kappa analysis of interobserver consistency confirmed good agreement among experienced and novice breast imagers for most descriptors within the various feature categories. Predicated on excellent, interpretable image quality, and acknowledging the importance of real-time ultrasound observations, BIRADS assessments are based on analysis of combined features—using descriptors from several feature categories rather than any single one, with the most suspicious feature dominating the assessment and recommendation. The use of multiple features in the ultrasound analysis of breast lesions allows greater diagnostic confidence which, in turn, can increase specificity. For example, starting with one feature category, shape, an oval mass might be cystic or solid, benign or malignant. An oval mass oriented parallel to the skin (‘wider-than-tall’) does not limit possibilities much—the mass could still be benign or malignant, cystic or solid. Add a descriptor from the margin category, and the specificity jumps up. If the margin is circumscribed, the likelihood of malignancy diminishes, and descriptors from additional categories, such as echogenicity and posterior features, can be added to refine diagnostic possibilities further. If the mass were anechoic and posterior acoustic enhancement present, the diagnosis of a simple cyst could be made. If the margin were not circumscribed. (e. g., had spicules and microlobulations), and posterior acoustic shadowing were added, the mass would be incompatible with a simple cyst, and the analysis would lead the interpreter toward cancer or one of its mimics, with biopsy as the management recommendation. Primary feature categories for diagnosis include shape, margins, and unique to ultrasound, orientation with respect to the skin. Additional feature categories that may help improve specificity include echogenicity, border zone, and posterior features as well as the BI-RADS category of “Effect on Surrounding Tissue.” The effect, or lack of effect, of the mass on the tissue surrounding it, can provide supporting evidence, and it is important to assess the breast anatomy around a mass for an echogenic halo, disruption of fat planes, and duct derangements. When there is no mass and the lesion is itself architectural distortion, a beaded, ectatic duct, and skin thickening or edema, the anatomy and clinical history may provide clues to the lesion’s etiology. In its feature analysis approach, BI-RADS–US employs similar methodology to that used for CT analysis of lung nodules, MRI for masses of the liver, and several imaging techniques for lesions of bone. As long as the definitions of terms are understood by the system’s users, this standardized method of feature analysis for any abnormality makes possible structured reporting, a concise communication in which the significant points are clearly indicated and the assessment is yoked to a management recommendation. It also facilitates data tracking for self-audit of successes and failures, and a summary of outcomes, important at a time when healthcare dollars are challenged and the mantra is “pay for performance.” It is important to emphasize that descriptors within each feature category are selected after evaluation of at least two perpendicular views confirming reality of a lesion. When the performer is the interpreter, the analytic method is applied to a real-time assessment of the mass. Currently in development are computer-assisted diagnosis systems for breast ultrasound based on segmentation and feature extraction, initially using one view but now working with two perpendicular views. Lesion detection systems used with mammography are more difficult to apply to ultrasound because the US window is quite small; to date, the algorithms for ultrasound are applicable only to masses already detected. Portions of BI-RADS–US are reproduced below with permission of the ACR. Definitions of the terms used and examples of each of the descriptors are provided. In assessing a lesion for diagnosis, in addition to analyzing the ultrasound images, correlation with mammography and MRI, if these examinations were done, physical findings, clinical history, the patient’s age and other risk factors are important to consider. In feature analysis for breast ultrasound, the most important feature categories are shape, margin, and orientation taken together, and the feature most associated with malignancy will dominate in the overall assessment. An example would be an oval mass, parallel to the skin, with circumscribed margin except where an acute angle is seen at one edge of the lesion. If the interpreter has not performed the scan, real-time observation would be indicated. If the angular margin is confirmed, it influences the assessment and management: greater likelihood of malignancy with biopsy indicated rather than follow-up. As in mammography, great variability in tissue composition is the norm. The background texture of the breast may affect sensitivity to lesion detection, although further study is warranted. – Fat lobules comprising the bulk of breast tissue. No discrete hypoechoic areas are present in the area scanned (Fig. 3.1). – Fibroglandular tissue comprising the bulk of breast tissue (Fig. 3.2). Heterogeneity can be focal or diffuse. Breast echotexture is characterized by multiple small areas of increased and decreased echogenicity. Shadowing may occur at interfaces of fat lobules and parenchyma. This pattern occurs in younger breasts and those with heterogeneously dense parenchyma seen mammographically. How and if this affects the sensitivity of sonography merits study, but technical maneuvers may help in interpretive issues of true lesion vs. pseudopathology (Fig. 3.3). Fig. 3.1 Homogeneous echotexture: fat. Predominantly fatty breast tissue with well-defined fat lobules, hyperechoic Cooper ligaments and other connective tissue elements (arrows). Only a thin layer of more echogenic fibroglandular tissue is present (*). Anechogenicity of the simple cyst within the hypoechoic fat easily perceived with a high dynamic range and appropriate gain and contrast settings. With permission from ACR BI-RADS, 4th ed., 2003. Fig. 3.2 Homogeneous echotexture: fibro-glandular. Beneath a thin layer of subcutaneous fat lobules lies a uniformly echogenic layer of fibroglandular tissue. With permission from ACR BI-RADS, 4th ed., 2003. Fig. 3.3 Heterogeneous background tissue. The admixture of echogenic fibroglandular elements and hypoechoic ducts may prevent recognition of a small mass or may cause a group of ducts or a small fat lobule to be misinterpreted as an abnormality. Small hypoechoic area (arrow) was recognized as normal anatomic structure at real time imaging. With permission from ACR BI-RADS, 4th ed., 2003. A mass occupies space and should be seen in two different projections. Masses can be distinguished from normal anatomic structures, such as ribs or fat lobules, using two or more projections and real-time scanning (Fig. 3.4 a, b; Fig. 3.5 a, b). Fig. 3.4 a, b Fat lobule. Depending on the plane of section, a fat lobule may resemble a benign solid mass, such as a fibroadenoma (a). The fat lobule will elongate in the perpendicular view (arrow), excluding a mass (b). With permission from ACRBI-RADS, 4th ed., 2003. Fig. 3.5 a, b Ribs. a Section through the short axis of a rib (*) depicts an oval, circumscribed, horizontally-oriented, shadowing mass that resembles a fibroadenoma. b Anatomy provides the clue: the rib lies behind the pectoral muscle, not a site for primary breast cancer. The perpendicular projection shows the elongated, curved rib. With permission from ACR BI-RADS, 4th ed., 2003. Fig. 3.6 a–c Oval, hyperechoic fibroadenoma. a Circumscribed oval mass, parallel to skin, noticeable only because of increased echogenicity (arrows). Its features all appear benign. b Perpendicular view shows mass surrounded by fat lobules (asterisks) except posteriorly, where it abuts a Cooper ligament. c Mammogram shows small mass correlating with US (arrow); the soft tissue density differentiates it from a lipoma, which would have fat density on the radiograph. Figures a and b with permission from ACR BI-RADS, 4th ed., 2003. This feature of masses is unique to ultrasound imaging. Orientation is defined with reference to the skin line. A parallel or “wider-than-tall” orientation is a property of some benign masses, notably fibroadenomas; however, many carcinomas also have this orientation. Shape and marginal characteristics should help dictate the level of suspicion of malignancy. Fig. 3.7 a, b Oval shape—macrolobulated. a Sagittal view of oval (macrolobulated) mass with circumscribed margins. b Transverse view of this mass is round. Oval masses viewed orthogonally to their long axes will be seen as round. Core biopsy confirmed fibroadenoma. With permission from ACR BIRADS, 4th ed., 2003. Fig. 3.8 a, b Round shape. Longitudinal (a) and transverse (b) views of a round complex cystic mass containing a small round, echogenic intracystic mass (arrow). The intracystic component is a thrombus that formed in a cyst that bled after aspiration. With permission from ACR BI-RADS, 4th ed., 2003. Fig. 3.9 a, b Irregular shape. Orthogonal views of a poorly differentiated infiltrating ductal carcinoma whose shape cannot be described by a geometric term. This irregular mass is hypoechoic compared with the surrounding fat lobules. Figure a with permission from ACR BI-RADS, 4th ed., 2003. Fig. 3.10 Orientation—parallel. Simple cyst. Radial view of oval, parallel (wider-than-tall), circumscribed mass demonstrating diagnostic criteria of a benign cyst: anechoic, oval (or round) and circumscribed with posterior enhancement. With permission from ACR BIRADS, 4th ed., 2003. Fig. 3.11 a, b Orientation—parallel. Orthogonal views of a palpable mass with spiculation and posterior shadowing show that the mass in longitudinal projection is horizontal (wider-than-tall) or parallel in orientation. Histologic diagnosis is infiltrating lobular carcinoma. Longitudinal view (a), transverse view (b). With permission from ACR BI-RADS, 4th ed., 2003. Fig. 3.12 Orientation—not parallel. Poorly differentiated invasive ductal carcinoma is diagnosis of vertically oriented oval mass whose long axis is not parallel to the skin line. Indistinct margins with short spicules anteriorly. At first glance the mass appears circumscribed, fibroadenomas will not have this orientation. Many poorly differentiated cancers demonstrate posterior enhancement rather than shadowing. With permission from ACR BI-RADS, 4th ed., 2003. The margin is the edge or border of the lesion. – Indistinct—No clear demarcation between a mass and its surrounding tissue. Boundary poorly defined (Fig. 3.14 a, b). – Angular—some or all of the margin has sharp corners, often forming acute angles (Fig. 3.15 a, b). – Microlobulated—short-cycle undulations impart a scalloped appearance to margin of mass (Fig. 3.16 a, b; 3.17 a, b). – Spiculated—the margin is formed or characterized by sharp lines projecting from the mass (Fig. 3.18 a, b). Fig. 3.13 a, b Margin—circumscribed. The margin of this intracystic papillary carcinoma is sharply defined at the interface of the cyst with the surrounding tissue. In a, the mass is pedunculated; in the orthogonal view (b
Development of the BI-RADS–US System
Basis of the BI-RADS–US System: Feature Analysis
Classification Categories in the BI-RADS–US System
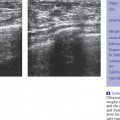
Background Echotexture
Homogeneous Background Echotexture
Heterogeneous Background
Masses
Shape
 Oval—a mass that is elliptical or egg-shaped (may include two or three undulations, i. e., “gently lobulated” or “macrolobulated”) (Fig. 3.6a–c; Fig. 3.7 a, b).
Oval—a mass that is elliptical or egg-shaped (may include two or three undulations, i. e., “gently lobulated” or “macrolobulated”) (Fig. 3.6a–c; Fig. 3.7 a, b).
 Round—a mass that is spherical, ball-shaped, circular, or globular. A round mass has an anteroposterior diameter equal to its transverse diameter (Fig. 3.8 a, b).
Round—a mass that is spherical, ball-shaped, circular, or globular. A round mass has an anteroposterior diameter equal to its transverse diameter (Fig. 3.8 a, b).
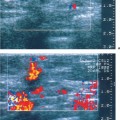
 Irregular—a mass neither round nor oval in shape (Fig. 3.9).
Irregular—a mass neither round nor oval in shape (Fig. 3.9).
Orientation
 Parallel—the long axis of a lesion parallels the skin line (“wider-than-tall” or horizontal orientation) (Figs. 3.10, 3.11 a, b).
Parallel—the long axis of a lesion parallels the skin line (“wider-than-tall” or horizontal orientation) (Figs. 3.10, 3.11 a, b).
 Not parallel—the anteroposterior or vertical dimension is greater than the transverse or horizontal dimension. These masses can also be obliquely oriented to the skin line. Round masses are not parallel in their orientation. Synonyms: “taller-than-wide” or vertical (Fig. 3.12).
Not parallel—the anteroposterior or vertical dimension is greater than the transverse or horizontal dimension. These masses can also be obliquely oriented to the skin line. Round masses are not parallel in their orientation. Synonyms: “taller-than-wide” or vertical (Fig. 3.12).
Margin
 Circumscribed—Margin well defined or sharp, with an abrupt transition between the lesion and surrounding tissue. Most circumscribed lesions have round or oval shapes (Fig. 3.13 a, b).
Circumscribed—Margin well defined or sharp, with an abrupt transition between the lesion and surrounding tissue. Most circumscribed lesions have round or oval shapes (Fig. 3.13 a, b).
 Not circumscribed—If the margin is not circumscribed, a mass has one or more of the following features: indistinct, angular, microlobulated, or spiculated. “Irregular” is not used to group these marginal attributes because irregular describes the shape of a mass.
Not circumscribed—If the margin is not circumscribed, a mass has one or more of the following features: indistinct, angular, microlobulated, or spiculated. “Irregular” is not used to group these marginal attributes because irregular describes the shape of a mass.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree