CHAPTER 22 Biliary system
Percutaneous invasive procedures involving the biliary system are designed to prove, characterize, and treat suspected biliary obstruction and, occasionally, biliary leaks. Huard provided the initial description of transhepatic cholangiography (THC) in 1937.1 Remolar and coworkers first described external percutaneous biliary drainage (PBD) in 1956.2 Nevertheless, these procedures did not become widely accepted until the late 1970s, when skinny-needle puncture techniques and coaxial exchange systems were developed to minimize the incidence of serious procedure-related hemorrhagic complications. Since their development in the late 1980s, endoscopic retrograde cholangiopancreatography (ERCP) and subsequently transendoscopic biliary drainage have diminished the need for THC and PBD. Percutaneous therapy continues to have a role in patients with anatomy unfavorable to endoscopic procedures and in institutions without skilled interventional endoscopists.3
Normal anatomy
The bile ducts of the posterior and anterior segments of the right hepatic lobe join near the porta hepatis to form the main right hepatic duct. The bile ducts of the medial and lateral segments of the left hepatic lobe merge to become the left main hepatic duct.4 The right posterior and right anterior segmental bile ducts join the left main hepatic duct directly in about 25% and 6% of individuals, respectively. The right and left main hepatic ducts coalesce in the hilum to form the common hepatic duct. At the porta hepatis, the bile ducts run anterior to the hepatic artery and portal vein. The gallbladder empties into the cystic duct, which is joined by the common hepatic duct to become the common bile duct (CBD), which travels within the hepatoduodenal ligament to drain through the sphincter of Oddi into the duodenum. A number of potential aberrant communications exists between the biliary tree and the gallbladder. The complex relationship between the biliary ductal system and the Couinaud hepatic segmental nomenclature has been described by Gazelle and colleagues.5
Transhepatic cholangiography
Patient selection and preparation
Given the widespread use of advanced cross-sectional imaging and ERCP, nearly all patients have THC in conjunction with PBD. The primary indication for THC is to confirm, localize, and characterize obstructive disease of the biliary system in patients with symptoms of pruritus or cholangitis who are not amenable to ERCP. 6 Increasingly, magnetic resonance (MR) cholangiography is being used to image the biliary system noninvasively.7,8 Occasionally, THC and PBD are used as part of the nonoperative management of patients with biliary leaks from bile duct injuries.
Most distal (periampullary) bile duct obstructions are managed endoscopically. Middle (extrahepatic and extrapancreatic) CBD lesions typically undergo operative resection with creation of a biliary-enteric anastomosis. Proximal (hilar) hepatic duct and intrahepatic bile duct strictures usually are treated with percutaneous techniques.
Bacterial overgrowth is common with biliary obstruction, particularly when caused by malignancy. Infectious complications are one of the common sources of fatal and major nonfatal complications. Biliary colonization with enteric organisms should be assumed in all patients with obstructive jaundice, whether evidence of suppurative cholangitis exists or not. Antibiotic prophylaxis is therefore routinely recommended.9–12 An antibiotic with appropriate coverage for gram-negative bacteria should be administered parenterally approximately 1 hour before the procedure to obtain adequate periprocedural blood levels.
The liver is a highly vascular organ, and the targeted bile ducts course adjacent to hepatic arteries and portal veins, which may be inadvertently injured. Coagulation factors (i.e., prothrombin time, partial thromboplastin time, platelet count, and, occasionally, the bleeding time) should be assessed and corrected if possible.13,14 A severe uncorrectable coagulopathy is a contraindication to the procedure. Massive, circumferential ascites impedes effective tamponade of blood or bile in addition to increasing the difficulty of catheter manipulation and the likelihood of accidental catheter dislodgment. These patients should have an alternative procedure or undergo paracentesis immediately before the transhepatic intervention. Uncooperative patients are also at high risk for bleeding complications for several reasons and should undergo an alternative procedure or receive general anesthesia.
Transhepatic biliary drainage procedures are significantly more painful than most diagnostic and interventional vascular procedures. Pain management should be liberal, particularly because multistaged procedures are common. Abundant local anesthesia and heavy conscious sedation, appropriately trained personnel, and monitoring equipment are essential. Frequently, assistance from anesthesia personnel is advisable and may include monitored analgesia (e.g., propofol and nitrous oxide) or general anesthesia.15 Intercostal nerve blocks can help with body wall pain. Most operators have found celiac plexus blocks to be cumbersome and ineffective.16
Technique
Skinny-needle (21 to 22 gauge) access into the intrahepatic bile ducts is the essence of THC and the first step of PBD. Access into the biliary system is gained through the right intrahepatic bile ducts using a low right intercostal approach or through the left intrahepatic bile ducts using an anterior subxiphoid entry (Fig. 22-1). The right-sided approach often is preferred, because most interventional radiologists are comfortable with the technique, the operator’s hands are not in the fluoroscopic field during guidewire and catheter manipulation, and the right lobe contains most of the hepatic parenchyma. Disadvantages include pain related to irritation of the rib periosteum, risk for crossing the parietal pleura (leading to bilothorax, empyema, and, rarely, pneumothorax), occasionally unfavorable angles for manipulation of catheters into the CBD, and the palmate-type (i.e., like a maple leaf), right-sided biliary branching pattern. This anatomic feature is more likely to result in isolated biliary segments in the setting of invasive hilar malignancy (Fig. 22-2).
Advantages to the left-sided approach include the ability to use ultrasound guidance to find a suitable bile duct while avoiding the adjacent vascular structures, the usually favorable angles for catheterization between the left-sided bile ducts and the CBD, and the more pinnate (i.e., like a feather) branching pattern typical of the left-sided bile duct (see Fig. 22-2), which is less likely to result in segmental isolation with invasive malignancy. Subxiphoid catheters avoid the pleura completely, usually are less uncomfortable than intercostal catheters, and are easier for the patient to flush. Disadvantages of left-sided access include increased radiation exposure to the radiologist’s hands and less experience by many interventional radiologists with this sometimes challenging technique. In patients with hilar malignancy that has resulted in isolation of right and left bile ducts, right- and left-sided biliary procedures should be planned, particularly when there is evidence of suppurative cholangitis.
Right-sided access involves placement of a skinny needle into the right-sided bile ducts from a low intercostal approach along the right mid-axillary line (see Fig. 22-1). The needle should enter below the 10th rib to avoid the parietal pleural reflection, although a more cephalad entry point frequently is required by the position of the inferior margin of the right lobe of the liver or the anticipated location of the porta hepatis. Fluoroscopy is used to assess the costophrenic sulcus during full inspiration to guarantee that the lung does not cross the projected access route. The needle is passed over the superior aspect of a given rib to minimize the risk for injury to the intercostal arteries. Under fluoroscopic guidance, the needle is then directed along a plane parallel to the tabletop and aimed toward the 11th thoracic vertebral body. If the same access may be used for biliary drainage, the needle pass should stop at approximately the right midclavicular line to avoid inadvertent puncture of left-side bile ducts, through which it would be nearly impossible to negotiate a guidewire or catheter toward the CBD.
The needle stylet is removed, and a small-volume syringe (e.g., 1 to 5 mL) with 60% strength contrast medium is attached. Small aliquots (e.g., 0.1 to 0.2 mL) of contrast material are delivered while the needle tip is slowly retracted under fluoroscopy. If the contrast material remains as a smudge at the needle tip, the tip is located in the liver parenchyma, and it should continue to be withdrawn. If contrast medium enters a tubular structure but then flows readily away, the needle tip probably resides within a vascular structure and withdrawal is continued. If contrast material fills a tubular structure and remains in the region of the needle tip, the tip probably is within the biliary system. Injection of an additional 5 to 10 mL should confirm the intrabiliary location and determine the ductal diameter and relationship to the porta hepatis and obstructive lesions.
Left-sided access originates from the left subcostal aspect of the epigastrium. Sonographic guidance permits direct visualization of the skinny needle as it is directed into an acceptable left bile duct while minimizing the likelihood of traversing the adjacent left hepatic artery or left portal vein branch (Fig. 22-3). Aspirated bile should be sent for routine Gram stain, culture, and sensitivity tests; if infectious complications arise, informed choices can be made regarding antimicrobial therapy.
If a diagnostic THC is to be performed without subsequent PBD, removal of 5 to 10 mL of bile alternating with instillation of 5 to 10 mL of contrast material permits diagnostic cholangiographic images while minimizing the risk of bacteremia and possible gram-negative sepsis. If a biliary leak is suspected, full-strength (60%) contrast material is warranted to visualize the fistula. However, if intraluminal filling defects (e.g., stones) or disease with subtle bile duct wall abnormalities (e.g., sclerosing cholangitis) is suspected, 60% contrast medium should be diluted by one half with normal saline solution. The biliary system should be aspirated before removal of the skinny needle if drainage is not planned.
Imaging findings
Most pathologic processes of the biliary tree are manifested in a few forms17–23
Table 22-1 Narrowing or Occlusion of the Biliary Ducts
| Disease or Cause | Suggestive Findings |
|---|---|
| Pseudostricture due to positional underfilling of contrast material (see Fig. 22-4) | |
| Pseudocalculus due to mucosal prolapse (see Fig. 22-5) | |
| Cholangiocarcinoma (see Fig. 22-6) | |
| Pancreatic or ampullary carcinoma (see Fig. 22-7) | |
| Extrinsic adenopathy | |
| Fibrosis (see Fig. 22-8) | Located at biliary-enteric or biliary-biliary anastomosis, site of injury, papilla, or in proximity to stones |
| Sclerosing cholangitis (see Fig. 22-9) | |
| Ischemic or AIDS-related cholangitis (see Fig. 22-10) | |
| Recurrent pyogenic cholangitis (Oriental cholangitis) (see Fig. 22-11) |
AIDS, acquired immunodeficiency syndrome; CBD, common bile duct.

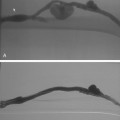
Figure 22-5 Mucosal prolapse. A, A polypoid filling defect is identified in the distal common bile duct (arrow).B, A few minutes later, the sphincter has opened (arrow).

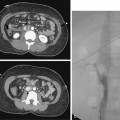
Figure 22-8 Expandable metallic stent for a benign tuberculous stricture. Contrast-enhanced computed tomography scan showed dilation of intrahepatic bile ducts bilaterally. The extrahepatic bile ducts were normal. A, Left anterior oblique view of a cholangiogram performed through a bilateral percutaneous biliary drainage catheter defined a near-occlusive eccentric stricture (arrows) of the hilar portion of the common bile duct, which had been retracted posteriorly, presumably because of fibrosis. B, After attempted percutaneous cholangioplasty, which failed because of elastic recoil, an 8-mm-diameter, 40-mm–long Wallstent (arrows) was deployed successfully. The left anterior oblique view documented excellent stent position and expansion.

Figure 22-10 Cholangioplasty for treatment of benign (ischemic) biliary stricture. A, Initial T-tube cholangiogram 1 week after orthotopic liver transplantation complicated by thrombosis of hepatic artery. Mild stricture of choledochal anastomosis (arrow) coexisted with diffuse narrowing of intrahepatic bile ducts. B, Five months later, left-sided percutaneous biliary drainage (PBD) was performed for obstructive jaundice and suppurative cholangitis. Diagnostic cholangiogram several days after PBD demonstrated a high-grade anastomotic stricture (arrow) with associated upstream bile duct dilation and numerous intraluminal filling defects from necrotic debris. C, Cholangiogram after dilation documented satisfactory expansion of stricture. D, After balloon cholangioplasty, a 12-Fr internal and external PBD catheter was left to stent the anastomosis. E, Endoscopic retrograde cholangiopancreatography 5 months after the cholangioplasty confirmed the anastomotic site was stricture free. Global dilation of common bile duct and central portions of intrahepatic bile ducts, various calibers of peripheral intrahepatic bile ducts, and copious intraluminal biliary debris are typical late findings of ischemic cholangiopathy.

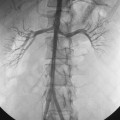
Figure 22-11 Staged multimodality management of complex biliary disease (recurrent pyogenic cholangitis). A, Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated multiple hilar biliary strictures (arrows), dilation of multiple intrahepatic bile ducts, and innumerable biliary calculi located within a massively dilated left bile duct. Pneumobilia resulted from prior endoscopic papillotomy. B, Magnified view of the abdominal computed tomography scan after the ERCP confirmed the hilar biliary strictures (arrows), bile duct dilation, and calculi. C, After bilateral biliary drainage, balloon cholangioplasty of the hilar biliary strictures, multiple sessions of basket retrieval of pigmented biliary calculi, and oral administration of ursodeoxycholic acid, the cholangiogram demonstrated improvement in the hilar biliary luminal diameter, partial resolution of the intrahepatic bile duct dilation, and near-complete removal of the calculi. D, After successful transluminal therapy, the bilateral percutaneous biliary drainage catheters have been converted to a long-term indwelling Silastic U-tube (arrows) to preserve transhepatic biliary access.
(Courtesy of Thomas Kinney, MD, and Horacio D’Agostino, MD, University of California, San Diego, Medical Center.)
Table 22-2 Dilated Biliary Ducts
| Disease or Cause | Suggestive Findings |
|---|---|
| Obstruction (see Figs. 22-7 and 22-8) | |
| Prior obstruction | |
| Choledochal cysts | |
| Type I (80%-90%) (see Fig. 22-12) | |
| Type II (2%) (see Fig. 22-13) | |
| Type III (1%-5%) | |
| Type IV | |
| Type V (see Fig. 22-14) | |
| Late phase ischemic injury (see Fig. 22-10) | |
| Denervation | |
| Oriental cholangiohepatitis (see Fig. 22-11) | Listed in Table 22-1 |
CBD, common bile duct.
* From Silverman SG, Coughin BF, Selzer SE, et al. Current use of screening laboratory tests before abdominal interventions: a survey of 603 radiologists. Radiology 1991;181:669.
Table 22-3 Biliary Duct Filling Defects
| Disease or Cause | Suggestive Findings |
|---|---|
| Air bubbles (see Fig. 22-15) | |
| Blood clot (see Fig. 22-16) | |
| Stones (see Fig. 22-17; see also Fig. 22-11) | |
| Pseudocalculus | Listed in Table 22-1 |
| Polypoid tumor | |
| Debris (see Fig. 22-10) | |
| Parasites |

Figure 22-16 Intrabiliary blood clot. After deployment and expansion of a Wallstent for a tuberculous biliary stricture, a cast of the biliary system caused by the blood clot is evident (arrows). A cholangiogram performed several days later showed spontaneous clearance of blood.
Table 22-4 Biliary Duct Leakage
| Disease or Cause | Suggestive Findings |
|---|---|
| Elevated intraluminal pressure (obstruction) (see Fig. 22-18) | |
| Poor ductal wall integrity | History of right upper quadrant surgery or injury, hepatic artery thrombosis, or irradiation |
| Bile duct injury | |
| Dehisced biliary anastomosis or cystic duct stump | |
| Ischemia | Associated with bilomas, intrahepatic or extrahepatic abscesses, ascites, and fistulas to bowel, skin, or wounds |
| Radiation therapy | May be associated with downstream strictures |
Biliary manometry is required occasionally to establish the presence of distal obstruction. The technique is modeled on the urodynamic Whitaker test.24,25 A baseline biliary pressure is established through the THC needle or through an external PBD catheter. A resting biliary pressure that exceeds 15 cm H2O is diagnostic for downstream obstruction. If the resting pressure is less than 15 cm H2O, a saline infusion challenge is indicated. Saline mixed with dilute contrast material is infused through a suitable infusion pump, initially at a rate of 2 mL/min and then at 4, 8, and 10 mL/min. After 5 to 10 minutes of infusion at each rate, the biliary pressure is recorded. If the biliary pressure exceeds 20 cm H2O at any point during saline infusion, a diagnosis of a significant downstream obstruction can be made. If the pressure does not exceed 20 cm H2O despite a saline infusion rate of 10 mL/min, a significant downstream occlusion is excluded.
Aspirated bile may be collected for cytologic examination. Cytologic material also can be obtained using a miniature brush biopsy device mounted on a guidewire26 (Fig. 22-19). The brush biopsy device is introduced through an angiographic catheter with the brush passed intraluminally to and fro across the stricture. The brush tip is then cut off and placed in a fixative solution. Another technique for obtaining cytologic material is fluoroscopically guided percutaneous skinny-needle aspiration (Fig. 22-20). Using the THC or PBD access, cholangiography is performed to localize the stricture. Through a second percutaneous approach (usually anterior subcostal), a skinny needle is advanced using fluoroscopic guidance to sample the identified stricture.
Currently available devices suitable for endoluminal biliary biopsy are flexible forceps developed for transvenous biopsy of the myocardium27 (see Fig. 22-6). The flexible transjugular liver biopsy needle (Cook Inc., Bloomington, Ind.) should not be used because it is designed to penetrate several centimeters beyond the lumen and may injure the adjacent hepatic artery or portal vein, possibly in an intraperitoneal location.