and Yuzou Sano2
(1)
Kyushu Research Forest, Graduate School of Agriculture, Kyushu University, Miyazaki, Kyushu, Japan
(2)
Laboratory of Wood Biology, Graduate School of Agriculture, Hokkaido University, Sapporo, Japan
Abstract
The protocol of freeze stabilization and cryopreparation techniques to examine the distribution of water in living tree stems by X-ray imaging and cryo-scanning electron microscopy have been developed and described. The brief procedures are as follows. Firstly, a portion of transpiring stem is frozen in the standing state with liquid nitrogen to stabilize the water that is present in the conducting tissue. After filling with liquid nitrogen, discs are then collected from the frozen portion of the stem and stored in liquid nitrogen. In a low-temperature room, the samples for X-ray imaging are sectioned with a fine handsaw, and trimmed sample blokes for cryo-scanning electron microscopy are cleanly planed using a sliding microtome. Finally, the frozen sections are irradiated in a soft X-ray apparatus, and the small blocks are examined in cryo-scanning electron microscope after freeze-etching and metal coating.
Key words
Freeze stabilizationCryoplaningX-ray imagingCryo-scanning electron microscopyWater distributionXylemPhloemPlant tissue1 Introduction
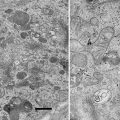
X-ray imaging and cryo-scanning electron microscopy (cryo-SEM) have been used to examine the frozen-hydrated plant tissues. X-ray imaging is useful for surveying a wide range of water distributions at tissue level [1–4]. X-ray imaging visualizes a transmission image of soft X-ray through the sample section (see Fig. 1). The intensity of X-ray on the imaging plane depends on the sample tissue density [5] and the water content [6], because the cell wall and water attenuate X-ray. Some studies represent the highly attenuated region a darker image (see Fig. 1a and also refs. 1, 7, 8) while other reports show it a brighter image [2–4, 9]. The cell wall and water distribution are clearly discriminated when the same sample is observed in both the green state (see Fig. 1b) and the dry state (see Fig. 1c). The resolution of X-ray imaging is sufficient to identify growth-ring boundary in conifers (see Fig. 1d and also refs. 2, 8) and in diffuse-porous hardwood species that have small vessels (see Fig. 1e). In some cases, each wide vessel can be observed (see Fig. 1f and also [7]).
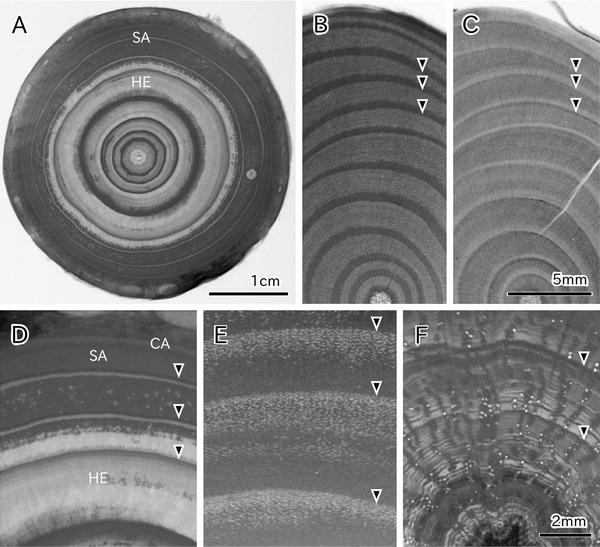

Fig. 1
Soft X-ray photographs showing cross sections of tree stems. (a) Abies firma, taken in green state. Difference in water content between sapwood and heartwood is apparent. (b and c) Pieris japonica, taken in green and dry states, respectively. It is identified that more water is contained in inner layer of each growth ring by comparing these images. (d) Part of a. It is apparent that dehydration occurs in the outer thin layers of the second and third rings and inner layers of the third and fourth rings. (e) Viburnum odoratissimum var. awabuki, taken in green state. Many vessels in the outermost layer of three growth rings are dehydrated. (f) Lithocarpus edulis, taken in green state. Water-filled vessels and cavitated vessels are recognizable. Sapwood (SA); heartwood (HE); cambial zone (CA); growth-ring boundary (arrowhead)
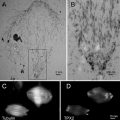
Cryo-SEM is useful for observing the state of cytoplasm at the cellular level without chemical fixation and dehydration [8, 10–14]. The presence or absence of water in the lumina of dead cells is clearly distinguished in each cell (see Fig. 2). To observe the dynamic state of a plant tissue by cryo-SEM, samples are usually prepared by cryo-planning (see Fig. 3a) or freeze-fracturing (see Fig. 3b and also [10, 11, 15, 16]). Freeze-fracturing is simple and permits the cellular ultrastructure of various plant tissues to be revealed [17–22]. However, freeze-fracturing is not adequate for examining the lumina of axial elements such as vessels and tracheids because it is difficult to cleanly expose a cross section of secondary xylem in a tree sample (see Fig. 3b). Although a longitudinal section can be cleanly exposed by freeze-fracture, the frequency of exposure of lumina of axial elements is low because the fracture tends to occur between cell walls of the adjacent cells. In addition, ragged surfaces often cause a substantial margin of error in quantitative morphological analyses. A series of suitable surfaces from the same specimen is usually impossible to obtain [16, 23].
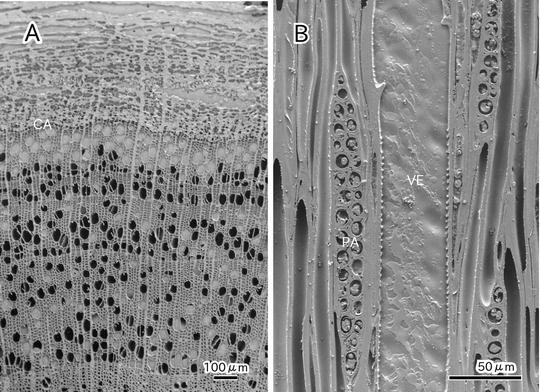
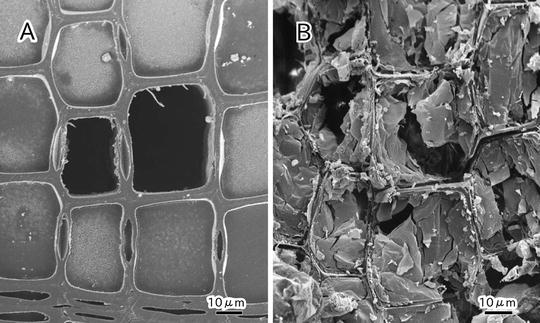

Fig. 2
Cryo-scanning electron micrographs showing a cross section of secondary xylem and phloem of Cornus sericea (a) and a tangential section of secondary xylem of Acer mono var. marmoratum f. dissectum (b). Cambial zone (CA); vessel (VE); parenchyma (PA)

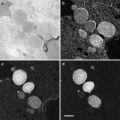
Fig. 3
Cryo-scanning electron micrographs of cross section of secondary xylem in Abies sachalinensis exposed by cryoplaning (a) and freeze-fracture (b)
In contrast, the cryoplaning method is useful for examining the state of lumina of axial elements [23]. With this method, 2–3 mm thick sections are cut away from the fractural surface of specimens with a steel blade on a sliding microtome under frozen conditions. It is possible to cleanly expose a wider range of surface of sample blocks (see Fig. 3a) and allows a desired smooth face of hard woody tissue to be cleanly exposed (see Fig. 2 and also [1, 24–27]).
This chapter describes a freeze stabilization and cryo-planning method that we devised. The method enables reproducible observations of the water distribution in the functional xylem of woody plant stems from tissue level to cellar level. In the text that follows, we will explain not only the sample preparation but also the whole process from the sampling procedure in the transpiring woody stems to X-ray imaging and cryo-SEM observation.
2 Materials
2.1 Freeze Stabilization and Collection of Samples
1.
Polyethylene funnel.
2.
Utility knife.
3.
Sticky (vinyl or duct) tape.
4.
Fat clay.
5.
Small Dewar flask.
6.
Large liquid nitrogen (LN2) Dewar flask.
7.
Handsaw.
8.
Cryostorage system.
9.
LN2.
2.2 Cryosectioning and Cryoplaning in the Low-Temperature Condition
1.
Low-temperature room or box maintained at −20 °C.
2.
Fine handsaw.
3.
Utility knife.
4.
Sliding microtome.
5.
Disposable blade for sliding microtome.
6.
Sample holder.
7.
Dewar flask.
8.
Glycerol.
9.
LN2.
2.3 X-Ray Imaging
3 Methods
3.1 Freeze Stabilization and Collection of Samples
1.
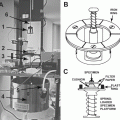
Cut a polyethylene funnel with a utility knife or clippers so that the diameter of the narrower-side hole of the cut funnel becomes slightly larger than that of a sample stem around which the cut funnel is fitted (see Fig. 4a).
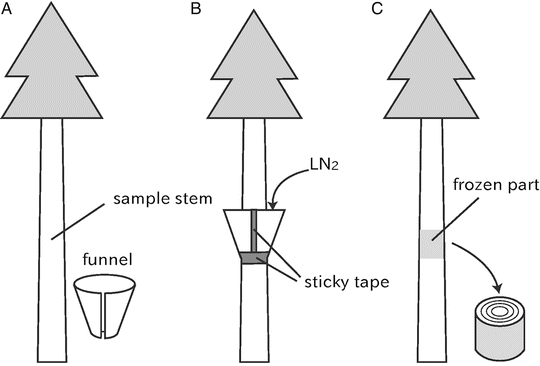

Fig. 4
Schematic diagrams of freeze stabilization techniques designed for woody plant stems
2.
3.
Additionally, pack the gap between the funnel and stem with plastic material such as fat clay.
4.
Run LN2 from an LN2 Dewar flask into a small Dewar flask.
5.
6.




Remove the collar after the freeze stabilization treatment and cut out 2–5 cm length discs with a handsaw from the frozen portion of the stem (see Fig. 4c).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree