FIGURE 18.1-1: Development of gastrointestinal tract showing the foregut, midgut, and hindgut along with the adult derivatives. The entire length of the endodermal gut tube is shown from the mouth to the anus. 1–4, pharyngeal pouches; AL, allantois; CA, celiac artery; CL, cloaca; DP, dorsal pancreas; E, esophagus; GB, gallbladder; HD, hepatic ducts; IMA, inferior mesenteric artery; LB, lung bud; SMA, superior mesenteric artery; ST, stomach; TD, thyroid diverticulum; VD, vitelline duct; VP, ventral pancreas; YS, yolk sac. (From Dudek RW, ed. High-Yield Systems: Gastrointestinal Tract. Philadelphia, PA: Lippincott Williams & Wilkins; 2010.)
The fetal gastrointestinal tract forms and undergoes its rotational process in the first trimester, returning into the abdomen by 11-weeks’ gestation. In the first trimester, meconium is produced from secretions of the liver and intestinal glands. Desquamated intestinal epithelium and amniotic fluid with meconium migrate from the small bowel to the rectum with increasing gestation age.
Fetal swallowing begins at 14 weeks’ gestation with the advent of bowel peristalsis. The fetus swallows 2 to 7 mL per day by 16 weeks’ gestation, 16 mL per day by 20 weeks, and 450 mL per day by term.1
THE NORMAL ABDOMEN
Ultrasound
Physiologic midgut herniation into the umbilical cord can be visualized as early as 8 weeks’ gestation with transvaginal scanning (see Fig. 18.2-5 in subchapter 18.2).2–4
Because of the greater supply of oxygenated blood to the left lobe of the liver, the left lobe is larger than the right in utero. The spleen may be visualized posterior to the stomach on the left. Normal measurements of the fetal liver, spleen, pancreas, stomach, gallbladder, and intestine are available (see Appendix tables 40–44).
Normally, the collapsed esophagus is not visualized by ultrasound (US).5 Fluid may be observed within the lumen of the small bowel by 13 weeks’ gestation.6,7 Meconium accumulates in the small and large bowel throughout the second and third trimesters.8,9 Bowel normally is collapsed and can be variably echogenic, at times similar in echogenicity to adjacent liver, spleen, and kidneys. Higher resolution linear transducers can be helpful in differentiating these organs. Linear transducers can make the bowel look unusually echogenic and must be carefully differentiated from truly echogenic bowel that can be associated with infections, IUGR, and cystic fibrosis (see section on meconium ileus) (Fig. 18.1-2).

FIGURE 18.1-2: Echogenic bowel at 25 weeks of gestation. Coronal US demonstrates abnormally echogenic bowel in a fetus with cystic fibrosis.
Swallowing occurs by 14 weeks’ gestation, so a fluid-filled stomach should always be detectable by the second and third trimesters.10,11 Echogenic debris can sometimes be seen layering in the stomach and can represent vernix, protein, or intra-amniotic hemorrhage.12 It is important to confirm situs (see section on heterotaxy in Chapter 16). An absent or small stomach on serial fetal US can suggest a mechanical obstruction such as esophageal atresia or neck mass; CNS anomalies or syndromes in which fetal swallowing is depressed; or oligohydramnios.
Fluid-filled bowel lumen is not seen in the first trimester, but begins to be noted by US by 20 weeks’ gestation. Peristalsis may be observed by 18 weeks’ gestation. Small bowel should not exceed 5 mm in diameter. The presence of dilated loops (>15 mm in length and 7 mm in diameter) suggests fetal bowel obstruction.13
Colon is best seen after 24 weeks’ gestation as hypoechoic regions along the periphery of the abdomen.8 The colon fills with meconium, enlarging throughout gestation measuring 3 to 5 mm at 20 weeks and by term measuring up to 28 mm in diameter.14 While variable, lumen diameter tables are available for gestational ages.4,14 At times, normal colon can be mistaken for dilated small bowel or masses.13
The prenatal US diagnosis of bowel atresia can be difficult and is dependent on the site of obstruction, presence of associated anomalies, and timing of the study. The prenatal detection rate for obstruction is highest with duodenal obstruction and lowest for esophageal (24%) and anal atresia (6% to 8%).15–17 There is poor sensitivity in the prenatal US diagnosis of large bowel lesions and misdiagnosis between small and large bowel obstruction.18 Overall prenatal detection rate of gastrointestinal obstruction by US has varied from 24% to 34%.16,17 With confounding variables such as maternal obesity; overlying gas and bone; transient normal variant; and late presentation, prenatal diagnosis and assessment of potential prognosis of fetuses with gastrointestinal abnormalities can be difficult by US.
Magnetic Resonance Imaging
Fetal bowel is well visualized by MRI and can be easily differentiated from adjacent liver, spleen, kidneys, bladder, and gallbladder.19–24
Fluid-filled small bowel loops are high signal on T2w and low signal on T1w, while meconium is low signal on T2w and high signal on T1w. Thus, MRI can be a useful tool to help characterize bowel anomalies.19,20,23,24
The gastrointestinal tract is progressively filled by swallowed amniotic fluid that is T2 hyperintense and T1 hypointense. The esophagus may be transiently seen with fluid (Fig. 18.1-3). The stomach and duodenum should always be filled with T2 hyperintense fluid.

FIGURE 18.1-3: Normal esophagus. Coronal T2w MR image of a fluid-filled esophagus at 33 weeks of gestation.
The jejunum should normally be high T2 and low T1 signal after 33 weeks’ gestation. The distal small bowel has a more variable appearance dependent on gestational age. Before 32 weeks, 50% of distal small bowel remains high signal on T1w owing to slow protein-rich meconium progression.19,25 T1 high signal may remain in proximal dilated loops because of lack of progression of meconium if bowel atresia is present or there is delayed peristalsis.23–25
By 20 weeks’ gestation, the rectum normally is of high T1w and low T2w signal owing to the protein and mineral content of the meconium accumulating near the functionally obstructed anus. The rectum should be posterior to the bladder and 1 cm below the bladder neck. The rectal anteroposterior diameter increases with gestational age, measuring 4 to 8 mm at 24 weeks and 9 to 15 mm by 35 weeks.19 Colonic luminal volume increases exponentially with gestational age. Colonic luminal volumes at 20 to 37 weeks’ gestational age range between 1.1 and 65 mL with greater variations later gestation.23
With advancing gestation, meconium not only distends the colon but also begins to fill the entire colon. The descending colon is filled with meconium by 24 weeks gestation (Fig. 18.1-4). However, there is greater variability proximally with only half of normal cases having meconium-filled ascending colon by 31 weeks’ gestation.19

FIGURE 18.1-4: Meconium. Coronal T1w MR image demonstrates high signal within the descending colon and rectosigmoid at 33 weeks of gestation.
BOWEL DISORDERS
The most common bowel abnormalities involve a mechanical or functional obstruction that results in a proximal bowel dilatation. Common causes of dilated bowel are listed in Table 18.1-1. At times, associated anomalies are present (Table 18.1-2). Dilated bowel loops may contain fluid or meconium. It is important to consider normal bowel in the differential when dilated bowel is thought to be present. Other intra-abdominal cystic masses should also be considered when a dilated loop of bowel is thought to be present. Differentiating dilated ureter from bowel may be difficult as well.
Etiology of Dilated Bowel
Esophagus Esophageal atresia Small bowel Jejunal atresia Ilial atresia Meconium ileus Enteric duplication Congenital diarrhea Hirschsprung disease Large bowel Hirschsprung disease Anorectal atresia Pitfalls Hydroureter Normal bowel/meconium |
It can take time for bowel dilatation to become apparent. Distal atresias such as anal atresia may never develop dilated bowel because of the absorption of fluid by the multiple proximal bowel loops. Jejunal and ilial atresias secondary to vascular ischemia may not develop until the second trimester, with bowel dilatation progressing only later in gestation. Conversely, proximal obstructions from duodenal atresia may be easily identified in the early second trimester.
Polyhydramnios can develop with bowel obstruction, but its presence is variable and its absence should not exclude the diagnosis. Esophageal atresias may present with early polyhydramnios, but if a tracheoesophageal fistula (TEF) accompanies the atresia, the presence of a fluid-filled stomach and normal amniotic fluid can result in a missed diagnosis prenatally. Proximal duodenal obstructions may only develop polyhydramnios in 50% of cases owing to the absorption of fluid within the dilated stomach and duodenum. It is common for more distal small and large bowel obstructions to have normal amniotic fluid volumes.26
The following sections will discuss the major bowel disorders that can be identified by US. MRI has become a useful adjunct in helping assess meconium location and further defining pathology. Accurate diagnosis allows for improved counseling, appropriate delivery planning, and delivery planning, and postnatal care.27–30
Proximal Gastrointestinal Disorders
Esophageal Atresia
Incidence: Esophageal atresia with or without TEF occurs in 1 in 2,500 to 4,000 live births. Males are slightly more affected than females.31
Embryology and Pathology: Tracheoesophageal anomalies are the result of incomplete division of the foregut into the ventral respiratory portion and dorsal digestive portion by the tracheoesophageal septum. At the 4th week of gestation, the growth of the ectodermal ridge is interrupted, resulting in a TEF.
There are five types of tracheoesophageal anomalies (see Fig. 17.3-4 in previous chapter):
Type A: Esophageal atresia with a TEF to the distal esophageal segment (>75%)
Type B: Esophageal atresia without TEF (10% to 15%)
Type C: H-type TEF with no esophageal atresia (5%)
Type D: Esophageal atresia with TEF to both the proximal and the distal esophageal segments (<5%)
Type E: Esophageal atresia with a TEF to the proximal esophageal segment (<5%)
Diagnosis
Ultrasound: Esophageal atresia, with or without a distal fistula, can be difficult to diagnose prenatally with sonographic detection rate for esophageal obstruction ranging from 24% to 30%.15,32–36 When a TEF is present, the sensitivity of US in prospectively making the diagnosis drops to 12%. Diagnosis relies on indirect sonographic findings including polyhydramnios and a persistently small or absent stomach but can result in a false-positive rate as high as 27%34 (Fig. 18.1-5).

FIGURE 18.1-5: Tracheoesophageal fistula in a 32-week-gestation fetus with two-vessel cord. A: Axial US demonstrates a small stomach (arrow). Polyhydramnios was present. B: Sagittal T2w MRI demonstrates a small amount of fluid in the proximal esophageal pouch (arrow). Following delivery, an esophageal atresia with distal TEF was diagnosed.
Associated anomalies suggesting VACTERL association (vertebral, renal, radial ray, cardiac, two-vessel cord) may help in suspecting this diagnosis prenatally.35
Type A isolated esophageal atresias are the most likely to be diagnosed prenatally by US. If there is failure to visualize the fetal stomach over several hours, the diagnosis of esophageal atresia should be considered, particularly in the presence of polyhydramnios. At times, a dilated proximal esophageal pouch may be noted in the neck or upper mediastinum.36–45
The high false-negative rate of identifying a TEF is due to the fact that the amniotic fluid from the proximal pouch can pass into the stomach through the tracheo-fistula with a resultant fluid-filled stomach present. Polyhydramnios develops in only one-third of these cases. The proximal pouch is more likely to be missed because of intermittent filling and emptying.37,39
If the stomach is small, TEF should be considered, particularly if polyhydramnios is also present. If there is marked polyhydramnios with a visualized stomach, and no additional findings, the diagnosis of TEF should be considered in the differential.
The presence of vertebral, cardiac, renal, limb anomalies, or two-vessel cord is suggestive of VACTERL. Up to 60% of individuals with VACTERL association have either esophageal atresia or TEF.43 Thus, in cases with one or two of the above findings, careful evaluation for possible TEF should be performed. The presence of polyhydramnios and small stomach in addition to a two-vessel cord is highly suggestive of a TEF and should be counseled appropriately.35
MRI: MRI can be a useful adjunct in the prenatal diagnosis of esophageal atresia. Dilated proximal esophageal pouches have been identified by MRI.19,21,46 However, this finding may be transient by both US and MR, and the esophagus can be collapsed throughout each study.
MRI is also useful in the identification of associated VACTERL anomalies such as pulmonary atresia, vertebral anomalies, and at times anal atresia (see later).
Differential Diagnosis: The absence of a fluid-filled stomach by US or MRI can be due to multiple etiologies. A repeat scan should be performed to confirm that the stomach has not simply emptied during the study.
If no normal stomach is identified, differential includes displacement of the stomach into the chest, or heterotaxy. Oligohydramnios is the most common cause of an empty stomach, and microgastria is the least common.
Oral and neck masses occluding the pharynx may result in a small or empty stomach. Sonographic observation of swallowing and muscular activity may help exclude a neuromuscular etiology.
Associated Findings: Up to 50% of patients with esophageal atresia have other anomalies. Cardiac malformations are the most common (25%) and result in the highest morbidity and mortality.47 Additional anomalies include gastrointestinal (28%), genitourinary (13%), musculoskeletal (11%), CNS (7%), and facial anomalies (6%).48
Gastrointestinal anomalies include malrotation, anorectal atresia, duodenal atresia, and annular pancreas.48 In Down syndrome, the combination of esophageal and duodenal atresia has been reported.49–53
The VACTERL association typically presents with several anomalies, including TEF (see section on VACTERL in Chapter 20).54
The risk of aneuploidy includes trisomy 18 and 21. Prenatal series report a wide incidence of aneuplody ranging from 6% to 44%.32,35,41 In a large postnatal series, however, chromosomal abnormalities were reported to be 5% of cases.51
Management: If the diagnosis of TEF is suspected, serial USs may be needed to confirm the diagnosis. Careful assessment of potential associated anomalies should be performed, including echocardiography. Karyotype should be performed because of the risk of chromosomal abnormalities, including trisomy 21 and 18.35
Polyhydramnios develops in up to 60% of cases of esophageal atresia/TEF, typically in the third trimester.32 Thus, follow-up studies to assess amniotic fluid volume are important because of the added risk of preterm labor with polyhydramnios. Bed rest, tocolytic agents, and reduction amniocenteses may be required. Betamethasone can be administered to increase fetal lung maturity if preterm labor develops.
Delivery should be planned at a center that has appropriate neonatal support. Caesarian section should be reserved for obstetric indications. If the esophageal atresia is confirmed postnatally, the infant should be transferred to a tertiary care center. Intravenous fluid should be administered. A sump tube should be placed in the proximal esophageal pouch to prevent aspiration.
Abdominal radiographs will demonstrate absent air in the bowel with esophageal atresia and associated spine anomalies. If there is a TEF, air typically will course into the stomach with normal appearing distal bowel.
Surgery is performed depending on the gestational age, birth weight, and presence of other anomalies. A decision is made as to whether a primary repair or a staged repair is to be executed. If the gap is long between the proximal and the distal pouch, a gastrostomy tube is placed for a staged repair. Growth of the two pouches typically occurs over several weeks and often results in successful reconstruction at 10 to 12 weeks’ gestation.
Prognosis: It is unclear whether the prenatal diagnosis of esophageal atresia/TEF actually improves the prognosis. Early diagnosis allows for appropriate counseling, screening for associated anomalies, and planned delivery at an appropriate center. Immediate supportive management may decrease the risk of aspiration.
Increased perinatal loss rates have been noted in several series because of the high incidence (60%) of associated anomalies.41,49,51
The overall prognosis depends on associated anomalies, gestational age, and respiratory complications. When there are no severe cardiac anomalies or chromosomal anomalies, survival is reported to be greater than 95% in infants over 2.5 kg.54 Infants may require mechanical ventilation, and prolonged hospital stays due to gastrointestinal issues. Short- and long-term follow-up is required after tracheoesophageal repair is performed to monitor possible complications such as leakage at the anastomosis or the development of esophageal strictures and gastrointestinal reflux.
Hiatal Hernia
Incidence: Rare in the prenatal and newborn period.
Pathology/Embryology: The gastroesophageal junction slides above the diaphragm through the esophageal hiatus.
Diagnosis: A dilated esophagus may be seen in the chest prenatally by both US and MRI.55–59 The stomach may be small.60,61 (Fig. 18.1-6).

FIGURE 18.1-6: Hiatal hernia. Coronal T2w MR image demonstrates a fluid-filled structure above the diaphragm (curved arrow) with a small infradiaphragmatic stomach consistent with a hiatal hernia.
Differential Diagnosis: Esophageal atresia may present with a dilated proximal esophageal pouch and small stomach mimicking a hiatal hernia. Esophageal duplication cyst could be considered in the differential. Hiatal hernia could be mistaken for a congenital diaphragmatic hernia.62
Associated Anomalies: Hiatal hernias have been associated with heterotaxy and Marfan syndrome.
Prognosis and Management: Isolated hiatal hernias typically have a good prognosis following surgical repair. There is a report of two giant hiatal hernias with associated anomalies having poor outcomes following delivery.63
Recurrence Risk: Familial cases have be reported rarely.64
Duodenal Atresia/Obstruction
Incidence: Duodenal atresia or stenosis is the most common type of intestinal atresia detected in the fetus occurring in 1 in 10,000 live births.27 Atresia is more common than stenosis occurring in up to 75% of cases.65 Approximately 30% of infants with duodenal atresia have trisomy 21.
Embryology/Pathology: The most common type of obstruction detected in the fetus is duodenal atresia or stenosis, thought to result from a failure of recanalization of the bowel at 8 to 10 weeks’ gestation. The blockage may be associated with an annular pancreas.
There are three types of duodenal atresia:
Type 1—Membranous mucosal atresia with an intact muscular wall (most common 69%)
Type 2—Short fibrous cord connecting two ends of the atretic duodenum
Type 3—Complete separation of the two ends associated with biliary tract anomalies65,66
Duodenal atresia can be associated with annular pancreas either by extrinsic compression or more commonly complete intrinsic atresia. The remaining 25% of cases are stenosis.
More than half of fetuses with duodenal atresia have associated anomalies. Trisomy 21 is the most frequent, occurring in up to 30% of cases. Increased incidence of duodenal atresia has been reported with gestational and preexisting diabetes, VACTERL association, and malrotation.67,68
Diagnosis
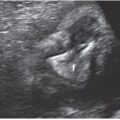
Ultrasound: Sonographic findings suggestive of duodenal atresia include a dilated fluid-filled stomach and proximal duodenum (fetal “double bubble”). The two structures should be contiguous to confirm the diagnosis (Fig. 18.1-7).69–71 A dilated duodenum that persists is abnormal. Prior to 24 weeks, the duodenum may not be sufficiently dilated to make the diagnosis.72–74 There are reports of the diagnosis being made in the first trimester.75 The diagnosis can be made in the early second trimester but is more commonly is made in the third trimester when the duodenum becomes more dilated.73,74 Polyhydramnios may develop in up to 50% of cases.69

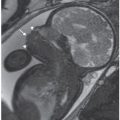
FIGURE 18.1-7: Duodenal atresia. A: Axial US demonstrates a fluid-filled double bubble at 28-week GA. B,C: T2w and T1w MR images, respectively, confirm the presence of a fluid-filled double bubble. D: Postnatal radiograph confirms the presence of an air-filled double bubble.
In cases of duodenal stenosis or associated esophageal atresia, the diagnosis can be missed prenatally if the duodenum does not become sufficiently dilated to recognize.76 At times, the stomach becomes extremely dilated, and the duodenal bulb may be difficult to visualize, resulting in a missed diagnosis.77
As 30% of fetuses with duodenal atresia have trisomy 21, other sonographic features of Down syndrome need to be searched for.72,78,79 Anomalies associated with trisomy 21 include small or absent nose, nuchal fold thickening, hypoplastic middle phalanx of the fifth digit, short humerus and femur, echogenic bowel, mild ventriculomegaly, mild pyelectasis, and cardiac anomalies such as AV canal and ventriculoseptal defects (see section on trisomy 21 in Chapter 20).
Duodenal atresia and stenosis can be part of the VACTERL association. In these cases, hemivertebra, radial ray, and renal anomalies may be identified.79 When a TEF and duodenal atresia are present together, a markedly dilated stomach may develop. If esophageal atresia and duodenal atresia are present together, the stomach may be small with a collapsed duodenum, making the diagnosis difficult to confirm prenatally.49,50,53,79
MRI: MRI can be used as an adjunct in the diagnosis of duodenal atresias. On T2w, the fluid-filled dilated stomach and duodenum will be of high signal with decompressed bowel loops distally (see Fig. 18.1-7B). The distal normal size small bowel loops of fluid signal and normal size colon and rectum filled with meconium should be easily visualized. Thus, multiple intestinal atresias which may mimic duodenal obstruction can be excluded by MRI if normal distal bowel is identified. The dilated duodenum is easily differentiated from adjacent gallbladder, and duplication cysts by MRI.18
Associated anomalies in the brain, spine, limbs, lung, and anal atresia may be confirmed and further evaluated by MRI.80
Differential Diagnosis: The differential diagnosis includes annular pancreas, malrotation with Ladd’s bands, duplication cysts, preduodenal portal vein, and choledochal cyst.76 Documenting that the fluid-filled structure is contiguous with the stomach can help differentiate duodenal atresia/stenosis from a duplication or choledochal cyst.81,82
Associated Anomalies: Duodenal atresia is an isolated finding in one-third to one-half of cases. However, it is often associated with other malformations, including cardiac, gastrointestinal (biliary atresia, agenesis of the gall bladder), renal, and vertebral anomalies.72,78,79
Thirty percent of newborns with duodenal atresia or stenosis have Down syndrome. Conversely, approximately 2.5% of patients with Down syndrome have duodenal atresia or stenosis.72
Prenatal Management: If the diagnosis of duodenal atresia or stenosis is suspected, serial USs may be needed to confirm the diagnosis. As 30% of fetuses with duodenal atresia have trisomy 21 and 20% to 30% have congenital heart disease, fetal karyotype analysis and detailed fetal survey, including fetal echocardiography, are indicated.83
Amniotic fluid digestion enzyme assays, including gamma glutamyl transpeptidase (GGTP), aminopeptidase (AMP), total alkaline phosphatase (ALP), and intestinal form (iALP) and total protein have been evaluated. Muller et al. correlated elevations of GGTP and iALP with duodenal and small bowel atresias. For duodenal atresia, sensitivity was reported to be 71% with a 100% specificity, while distal bowel sensitivity was 69% and 83% specific.20,84 Normal values in amniotic fluid do not exclude the diagnosis of obstructions.
As duodenal atresia can be complicated by polyhydramnios in up to 50% of cases69,83 follow-up sonograms are useful to track amniotic fluid volume. Polyhydramnios can result in preterm labor, so close monitoring is useful. Prenatal pediatric surgical consultation with parents can be helpful in alleviating anxiety and planning postnatal care.
The fetus will usually not need delivery at a high-risk center, and can be safely transported after birth to a pediatric center after enteric tube decompression of the intestinal tract to prevent aspiration of gastric contents. Intravenous fluid should be administered to replace volume with attention to electrolyte imbalance.
Abdominal radiographs will demonstrate the typical air-filled “double bubble” with absent gas distally (see Fig. 18.1-7D). If there is a stenosis or the biliary tree bridges the atresia, air may be traversed distally into the small bowel. A postnatal double bubble radiographic appearance may also be seen in the setting of malrotation of the midgut with or without associated volvulus.
Surgery is typically performed on the day of the delivery after assessment of other associated anomalies, and volume resuscitation and electrolyte imbalances are corrected.
Prognosis/Outcome: Several series suggest that prenatal diagnosis does reduce morbidity and shortens hospitalization.85–87 Survival has improved over the past two decades, with a 95% survival reported.76,78,88,89 Outcome now is more related to associated anomalies, particularly cardiac defects.83 Late complications include dysmotility of the proximal duodenum resulting in reflux, gastritis, pancreatitis, and/or cholecystitis.89
Jejunal and Ileal Atresia
Incidence: The incidence of small bowel atresia is 1 per 1,500 to 5,000 births.29,90 Atresias can occur anywhere in the small bowel but most commonly present in the proximal jejunal (30%) and distal ileum (35%) with multiple atresias are found in up to 6% of cases.28,91
Embryology/Pathology: Jejunal and ileal atresia are a complete obstruction of the bowel lumen and are more common than small bowel stenosis. Small bowel atresias are thought to be caused by vascular ischemia in the second trimester with the ischemic necrosis leading to resorption of the segment or fibrous scarring.92–94
Type 1—32%; lumen is obstructed by an intact diaphragm or membrane made of mucosa and submucosa. The muscularis and serosa are intact with continuity of the proximal and distal segments. If the membrane stretches, it takes the shape of a windsock.
Type 2—25%; fibrotic cord connecting two blind-ending bowel segments
Type 3
3a—15%; complete separation of blind-ending loops
3b—11%; mesenteric defect with large gap in small bowel mesentery. The distal small bowel is short and coiled like an apple peel. Terminal ileum is perfused from a single ileocolic artery (can be familial)
Type 4—6%; multiple atresias95,96
Lesions can range from a focal atresia to complete atresia of the entire herniated bowel possibly related to constriction/volvulus.97 In these cases, the necrotic bowel is resorbed with the abdominal wall defect closing as the herniated bowel involutes. The prognosis is poor because of loss of most of the small bowel. Placental vascular abnormalities have also been associated with small bowel atresias.93 Atresias have been reproduced in experimental animals by ligation of mesenteric blood vessels.98 Maternal use of vasoconstrictive medications may contribute to interruption of mesenteric blood flow.99 Inherited thrombophilia leading to spontaneous thrombosis may play a role in some cases.100
Gastroschisis have been associated with bowel atresia because of ischemic injury from bowel kinking.101–104
The incidence of small bowel atresia secondary to meconium ileus is reported to be 10% of cases. The incidence of associated aneuploidy and extraintestinal anomalies is low.
Diagnosis
Ultrasound: Obstruction of the fetal bowel may be sonographically diagnosed when dilated bowel loops are detected proximal to a point of obstruction. Hyperperistalsis of the proximal bowel may be observed. The diagnosis of obstruction before 18 weeks’ gestation is rare and may be difficult to diagnose before 24 weeks’ gestation. Bowel loops become progressively more dilated with vigorous peristalsis in the third trimester.
The presence of dilated loops (>15 mm in length and 7 mm in diameter) and/or mural thickness of greater than 3 mm is most suggestive of fetal bowel obstruction.4,105,106 The abdomen can be distended with asymmetric increase in the abdominal circumference. A proximal or jejunal atresia may show a sonographic fetal “triple bubble” representing the dilated stomach, duodenum, and proximal jejunum (Figs. 18.1-8A and 18.1-9A). The jejunum’s capacity to dilate is greater than that of the ileum, and is therefore less likely to be associated with the complications of perforation, such as meconium peritonitis and meconium pseudocyst. Multiple dilated loops of bowel are more suggestive of a distal obstruction such as an ileal atresia. If meconium ileus is present, increased bowel echogenicity may be noted.

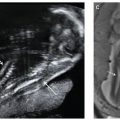
FIGURE 18.1-8: Jejunal atresia with fluid-filled dilated jejunal loops. A: Axial US image demonstrates a fluid-filled dilated loop of bowel. B: Coronal T1w MR image demonstrates a dilated fluid-filled loop of bowel containing no high-signal meconium. C: Postnatal radiograph demonstrates air in the stomach and proximal jejunum. D: Surgical image of jejunal atresia.

FIGURE 18.1-9: Jejunal atresia with meconium-filled dilated jejunal loops. A: Axial US image demonstrates a dilated loop of bowel filled with echogenic material. B: Coronal T1w MR image demonstrates dilated high-signal meconium-filled loops of small bowel. At surgery, jejunal atresia was confirmed.
Bowel distal to the level of obstruction is difficult to assess by US. Thus, differentiating an isolated atresia from multiple atresias is difficult sonographically.16,107
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree