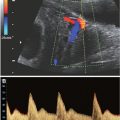
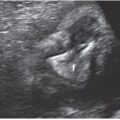
FIGURE 11.1: Monozygotic versus dizygotic twins. All dizygotic twins have dichorionic–diamniotic placentation. Monozygotic twins may form dichorionic–diamniotic, monochorionic–diamniotic, or monochorionic–monoamniotic placentation.
Definition/Incidence
Monozygotic twins, approximately one-third of twin gestations, result from ovulation and fertilization of single oocyte and subsequent division of the zygote into two fetuses with either mono- or dichorionic placentation (Fig. 11.2). During embryogenesis, dichorionic diamniotic twins split on days 2 to 3, monochorionic diamniotic twinning occurs between days 3 and 7, monochorionic monoamnitoic twinning occurs from days 7 to 14, and conjoined twinning occurs between days 14 and 15.18 The incidence of monozygotic birth is fairly constant, accounting for 1 in 330 of spontaneous live births, or about 1 in 160 babies.19–21 Of these, 70% are concordant healthy monozygotic twins and 30% are dizygotic19–21 (Fig. 11.3). Monzygotic twins have increased risks of complications such as twin reverse arterial perfusion syndrome (TRAP), conjoined twins, and twin-to-twin transfusion.

FIGURE 11.2: Position of the placenta in dichorionic and monochorionic placentation. A: Dichorionic–diamniotic placentation with two separate placentas. B: Dichorionic–diamniotic placentation with a fused placenta. C: Monochorionic–diamniotic placentation with a single placenta. D: Monochorionic–monoamniotic placentation with a single placenta.

FIGURE 11.3: Frequency of dichorionic and monochorionic placentation with regard to zygosity and fetal sex.
Dizygotic twins represent two-thirds of twin gestations, and occur from ovulation and fertilization of two oocytes (see Fig. 11.1), almost always resulting in a dichorionic placentation (see Fig. 11.2). Dizygotic twinning is a type of superfecundation in which more than one fertilized egg is present in the uterus. The spontaneous prevalence of live-born dizygotic twinning in North America and Britain is 1 in 100 births,20 but incidence varies widely by region (see Fig. 11.3). Hereditary tendency may increase the risk three times normal population.
Diagnosis
Zygosity can be determined by placentation or biochemical testing such as blood types, enzyme polymorphisms, and HLA types.22–25 Regarding placentation, a monochorionic placenta is monozygotic until proven otherwise. On the other hand, DNA typing is considered the most useful and reliable way of defining zygosity.26 However, because 70% of monozygotic twins share vascular placental connections, DNA in blood cells may appear to be identical even if the twin pair are genetically or epigenetically discordant.25,26
Classification
Twins can be classified clinically via chorionicity or genetically based on zygosity. Controversy persists on whether outcomes of twin gestations are best assessed by zygosity or chorionicity. In the United Kingdom, a prospective observational study was conducted to compare fetal outcome based on chorionicity or zygosity. The authors, Carroll et al., used umbilical cord blood with microsatellite markers and placenta histology to determine zygosity and chorionicity, respectively. Carroll et al.27 reported that fetal outcomes in twin pregnancies are better predicted by chorionicity rather than zyosity. Caroll et al. also reported misclassification between genetic testing and clinical diagnosis of chorionicity. Similarly, Spiegler et al.28 reported significantly higher rates of clinical misclassification in monozygotic twins when compared with dizygotic twins (37% and 11%, respectively). Several studies have reported perinatal outcomes based on both clinical and genetic zygosity (Table 11.1).28,29 When defining outcomes based on chorionicity, monochorionic monoamniotic pregnancies have the highest mortality rate at 50%, followed by monochorionic diamniotic at 26%, and finally dichorionic diamniotic at 9%.
Determining Chorionicity
Early sonographic documentation of the number of placentas, or chorionicity, is important for optimal management of twin gestations. Evaluating the number of sacs and amniotic membrane also provides clues to chorionicity. In a dichorionic gestation, the amniotic membrane is thick as it is composed of two layers of amnion and two interposed layers of chorion. In monochorionic twins, the membrane is thin, composed only of amnions from each gestational sac. As the pregnancy continues, images of the placenta, sacs, and membranes become more difficult to obtain.30–32 Some authors have reported that the chorionicity, fetuses, and amnions can be documented by 5, 6, and 8 weeks, respectively.33 Most attest that accuracy is highest if the assessment of chorionicity is undertaken before 14 weeks’ gestation.34 In a study of 131 twin pregnancies, Stenhouse et al.34 reported ultrasound sensitivity of 77% for monochorionicity and 90% for dichorionicity after 14 weeks, whereas 99% accuracy was achieved for both groups before 14 weeks. With a composite of second trimester ultrasound markers (i.e., number of placentas, fetal phenotype, membrane thickness, and twin peak sign), the sensitivity and specificity for correct identification of monochorionic pregnancies is reported at 91.7% and 97.3%, respectively.35 Sometimes, there are cases of twin pregnancies where chorionicity can be difficult to determine. In these mothers, the pregnancy can be diagnosed as dichorionic without other ultrasound parameters if the fetuses differ in sex.33 Providers are cautioned against diagnosing a dichorionic pregnancy based on visualization of two placentas, especially during the second and third trimesters, because the “second” placenta could represent a succenturiate lobe.33 When chorionicity cannot be determined even with available diagnostics, the provider should consider sending the placenta to pathology after delivery.
Diagnosis
Imaging
Before 10 Weeks
Markers for chorionicity include the number of gestational sacs, amniotic sacs within chorioinic cavity, and yolk sacs. A strong relationship exists between the number of gestational sacs and chorionicity. Two gestational sacs and two embryo poles with fetal cardiac activity are suggestive of a dichorioinic twin pregnancy, whereas one sac with two embryo poles is most consistent with monochorionicity (Fig. 11.4).36 Use of transvaginal ultrasound is helpful before 10 weeks to identify the number of amniotic sacs. Similarly, the number of yolk sacs may help in the diagnosis of amnionicity: for example, two yolk sacs are suggestive of a diamniotic pregnancy (Fig. 11.5).37 When these findings are no longer present after 10 weeks, later sonographic findings become standard.

FIGURE 11.4: Dichorionic and monochorionic twins, first trimester. First-trimester examples of dichorionic (A) and monochorionic (B) twin gestations. Note the thick separating membranes of dichorionic placentation.
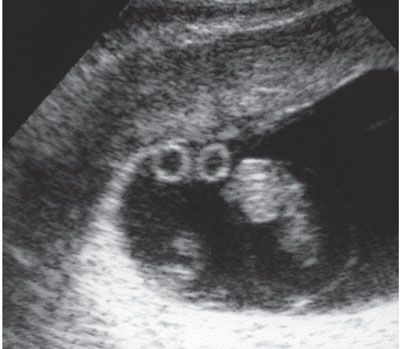
FIGURE 11.5: Monochorionic–diamniotic placentation with two yolk sacs. Ultrasound image of a monochorionic twin pregnancy in which there was difficulty in identification of the intertwin membrane shows two discrete yolk sacs, confirming a diamniotic gestation.
After 10 Weeks
Lambda sign: The lambda sign is a histologic feature of dichorionicity, representing extension of placental tissue into the base of the intertwine membrane (Fig. 11.6). The presence of a lambda or twin peak sign is diagnostic of a dichorionic pregnancy (Fig. 11.7).33 The lambda sign, which refers to the shape of the lateral edges of the membranes, was first described by Bessis and Papernik35 in 1981 and later by Finberg38 in 1992. In the second trimester, the twin peak sign becomes more difficult to visualize and disappears in about 7% of dichorionic pregnancies between 16 and 20 weeks.39–41 Therefore, the absence of this sign in the second or third trimester cannot exclude dichorionicity.39
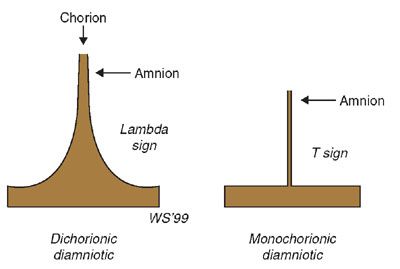
FIGURE 11.6: Schematic illustrating the lambda, or twin peak, and T signs observed in dichorionic and monochorionic twin pregnancies, respectively. In a dichorionic pregnancy with fused placentas, both the amnions and the chorions reflect away from the placental surface, creating a potential space into which villi can grow. This is called the lambda or twin peak sign. Monochorionic–diamniotic pregnancies have a single layer of continuous chorion limiting villous growth. This is called the T sign.

FIGURE 11.7: Dichorionic placentation. Ultrasound image of intertwin membrane–placental junction in a dichorionic twin gestation shows extension of the placental tissue into the base of the intertwin membrane (the lambda sign).
T sign: In contrast, monochorionic placentation is characterized as a single placental mass and a thin separating membrane, which is optimally detected when the membrane is oriented perpendicular to the ultrasound beam (see Fig. 11.6). This membrane intersects the placental junction with a “T” sign (Fig. 11.8). Although the T-sign generally indicates a monochorionic/diamniotic pregnancy,33 other instances can occur when this sign is not diagnostic. For example, as a dichorionic pregnancy progresses, the hallmark lambda sign may not be visible and can simulate a T configuration. This reiterates the importance of early ultrasound diagnosis of chorionicity.

FIGURE 11.8: Monochorionic placentation. Transabdominal ultrasound of a monochorionic twin pregnancy shows absence of the lambda sign and the presence of the T sign with the intertwin membrane abruptly joining the placental surface with no intervening chorion.
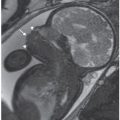
Thickness of intertwin membrane: Several authors have measured the thickness of the intertwin membrane as a tool for diagnosing chorionicity.33 In 1989, Winn et al.42 reported that during second trimester, the mean thickness of the intertwin membrane was 2.4 mm for dichorionic pregnancies and 1.4 mm for monochorionic pregnancies with an accuracy in predicting monochorionic or dichorionic twinning of 82% and 95%, respectively. Clinically, however, this has been difficult to apply as while the mean for dichorionic and monochorionic pregnancies differs, these dimensions can overlap for each type of placentation. Because it is not universally applicable, this measurement is not routinely used in clinical practice. In general, an intertwin membrane thickness of >2 mm indicates dichorionicity with a positive predictive value of 95%, whereas a thickness ≤2 mm indicates monochorionicity with a positive predictive value of 90% (Fig. 11.9).32 On fetal MRI, differences in membrane thickness may also provide clues to chronicity (Fig. 11.10).

FIGURE 11.9: Dichorionic twin. In this ultrasound photograph of a section of intertwin membrane in a dichorionic twin pregnancy, the intertwin membrane is thick and echogenic and three layers are seen, confirming dichorionicity.
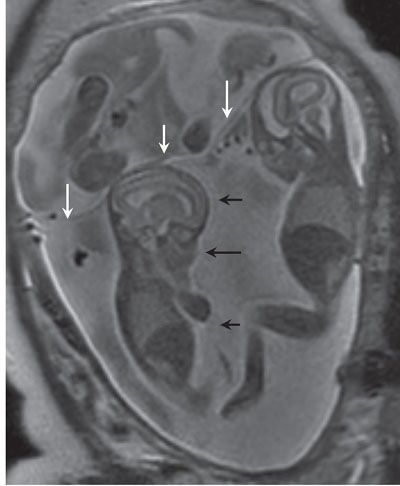
FIGURE 11.10: Membranes on Fetal MRI. Coronal SSFSE T2 image in a dichorionic triamniotic triplet gestation. The membrane separating the chorions is thick (white arrows) versus that separating the twins which share a placenta (black arrows).
Layers of intertwin membrane: In the second trimester, the number of membranes may be counted to determine chorionicity, and if there are >2, then dichorionicity is strongly suggested (see Fig. 11.9).33,43 This technique requires high-powered ultrasound technology that is not routinely available in clinical practice. Using this technique, researchers noted that visualization of four membranes indicated a dichorionic pregnancy, and two membranes a monochorionic pregnancy.
Prenatal Diagnosis: Prenatal diagnosis evolved tremendously in the past century in response to increasing pregnancies with higher maternal ages and thus an associated increased risk of Down syndrome. Prenatal diagnosis shifted from a risk assessment based solely on the mother’s age (i.e., medical history) to a quantitative risk assessment that evolved from the measurement of maternal alpha-fetoprotein (AFP) to that of human chorionic gonadotropin levels later during that decade.44 In the 1990s, nuchal translucency (NT) was introduced as another first trimester screening tool.45 In 1997, Lo et al.46 revolutionized prenatal screening and diagnosis with their discovery of cell-free fetal DNA, which is now commercially available. Each of these diagnostic markers has been validated first in singletons and then extrapolated to include twins and other higher order multiples. Twin and multiple gestations present unique challenges in prenatal screening and diagnosis.
MULTIPLE GESTATIONS AND CONGENITAL MALFORMATION
Anomalies that occur with increased frequency in monozygotic twins include hydrocephalus, anencephaly, holoprosencephaly, and sacrococcygeal teratoma. For any given defect, the pregnancy may be concordant or discordant in terms of the presence, type, and severity of the abnormality. However, the majority (80% to 90%) of structural defects are discordant regardless of zygosity.
Incidence
The overall prevalence of structural defects is 1.2 to 2.0 times higher in fetuses from twin pregnancies compared with singletons, with most of the excess risk due to increased rates in monochorionic twins.47 Monozygotic twins have two to three times higher rates of congenital anomalies than singletons or dizygotic twins.
Pathogenesis/Risk
Zygosity, rather than chorionicity, determines the risk of chromosomal abnormalities and whether or not the fetuses may be concordant or discordant for the anomaly. In dizygotic pregnancies, each twin has an independent risk for aneuploidy with the maternal age-related risk for chromosomal abnormalities for each twin being the same as in singleton pregnancies. Therefore, the chance of at least one fetus in a dizygotic twin gestation being affected by a chromosomal defect is twice as high as in singleton pregnancies (if zygosity is unknown, multiply singleton risk by 5/3). For these twins, the pregnancy-specific risk is based on the sum of the individual risk estimates for each fetus. In monozygotic twins, the risk for chromosomal abnormalities in each fetus is the same as in singleton pregnancies.48
Given the higher rates of congenital abnormalities in twins and also higher order pregnancies, understanding how multiples affect the sensitivity and specificity of the various prenatal screening and diagnostic tests is crucial. Clinicians should consider that although there are prenatal diagnostic options for twins and multiple gestations, screening accuracy is lower than for a single pregnancy.49
Risk for fetal aneuploidy is associated with advancing maternal age.50 In patients with singletons, advanced maternal age is defined as older than 35 years on the expected date of delivery.51 This designation is applied to women with a singleton or monochorionic twins. However, defining advanced maternal age is far more complex in women with dizygotic twins, triplets, and high-order multiples. In dizygotic twins, the risk of one fetus having an aneuploidy is twice that for a woman of the same age with a singleton.52
Diagnosis
First Trimester Screening
Ultrasound: In a retrospective study to evaluate the accuracy of antenatal ultrasound in the detection of fetal anomalies in 245 twin pregnancies, Edwards et al.51 reported overall prevalence rates of 4.9%. Antepartum ultrasound had a sensitivity of 88% and a specificity of 100% for the detection of anomalies in these patients, with a positive predictive value of 100% and a negative predictive value of 99%. Despite these findings, data are insufficient to make recommendations about frequency of anatomical ultrasound in multiple gestations.
Nuchal translucency is a noninvasive ultrasound technique that can be used between 11.0 and 13.6 weeks to detect aneuploidy. Although biochemical results vary according to the number of gestations, gestational age, and other factors, NT is an effective choice because it provides direct visualization of the embryo.51,53 Screening can be technically challenging in multiple gestations; however, if the nuchal region is visible for each fetus, the test can be effective. In evaluating NT in 448 twin pregnancies (both dichorionic and monochorionic), Sebire et al.54 reported that 7.3% of normal fetuses had an elevated NT above the 95th percentile. In 88.4% of twin pregnancies, both fetuses had a normal NT. The overall sensitivity of 88% was comparable with the singleton detection rate. The screen false positive rate was higher in monochorionic twins at 8.4% than in dichorionic twins at 5.4%. In multiples, risk assessment using first trimester methods can be calculated for each fetus or for the entire pregnancy. In monochorionic twins, each fetus has the same risk of being affected with Down syndrome. Thus, a single risk estimate for the duration of pregnancy is based on the average of the NT measurements. Each fetus in a dichorionic twin pregnancy is treated as a separate individual, and the risk for each fetus is calculated by using published NT values for singletons.55,56
Another interesting application of NT in monochorionic twins is the ability to predict twin-to-twin transfusion syndrome (TTTS).57,58 A NT threshold at the 95th percentile had a positive and a negative predictive value of 43% and 91%, respectively.57
Biochemical Markers
Pregnancy-Associated Placental Protein (PAPP-A): One screening tool for aneuploidy is the PAPP-A, a molecule that is secreted by the placenta. In singleton pregnancies, low levels of PAPP-A occur in fetuses with Down syndrome. However, compared with singleton pregnancies, PAPP-A levels are nearly double in normal twin pregnancies.59 The magnitude of the value depends on chorionicity so that PAPP-A is higher in dichorionic than in monochorionic twins.60 In general, maternal analyte levels can be adjusted for twin pregnancy; however, the detection rate for Down syndrome is lower than in singleton pregnancy (e.g., 93% detection in MC twins, 78% detection in DC twins, and 95% detection in singletons).61 In addition, maternal analyte levels for Down syndrome screening in twin pregnancy may be affected by early loss of one embryo of a triplet gestation.62,63
Beta-Human Chorionic Gonadotropin (β-hCG): It is a hormone that is also secreted from the placental trophoblast. Compared with singletons, β-hCG levels are approximately double in twins compared with singletons.59 Similar to PAPP-A, levels of β-hCG are lower in monochorionic than in dichorionic pregnancies.60 These values are also affected by the mode of conception, whether spontaneous or ART.
Combined Screening Using Ultrasound and Serum Analyte: In singletons, first trimester combined screening, which includes maternal age, NT, and maternal serum-free β-hCG and PAPP-A levels, has been shown to detect approximately 82% of cases of Down syndrome, with a 5% false positive rate.64 Wald et al.56 reported the detection with a 5% false positive rate for Down syndrome in monochorionic, dichorionic, and all twins as 73%, 68%, and 69% for NT alone and 84%, 70%, and 72% for combined tests, respectively. The accuracy of combined testing in the detection of Down syndrome approximated to singleton rates.
Karyotyping/Invasive Procedures: Screening detection rates for twin pregnancies are estimated to be 15% less than for singleton pregnancies.59 This can be explained at least in part as being due to the chemicals produced by the chromosomally normal fetus and abnormal fetus being averaged; thus, a total reported number may cause a normal test result. For these reasons, both fetuses of a dizygotic pair should undergo karyotyping unlike monozygotic twins.
Chorionic villus sampling: Chorionic villus sampling is a diagnostic test performed at 10 to 12 weeks of gestation to obtain placental cells for prenatal diagnosis. The procedure can be performed transcervically or transabdominally. Before samples are collected, an ultrasound is obtained to confirm the number of placentas and fetuses and determine chorionicity. In a transcervical technique, the patient is prepped, a sterile speculum is placed, and a small catheter is placed through the cervix under direct ultrasound visualization to obtain chorionic villi. Although some surgeons prefer biopsy forceps rather than a catheter under negative pressure, either technique can be used. In an abdominal approach, the abdomen is prepped, and a needle is inserted through the abdominal wall into the placenta, cells are aspirated, and then the needle is withdrawn. Only one chorionic villus sample is traditionally needed for monochorionic pregnancies. However, there are case reports of genetically discordant monochorionic twins, and thus two samples can be required. For dichorionic pregnancies, two separate placental samples are needed. If access to both placentas can be achieved via a transcervical approach without contamination, one could choose this method. However, if the second placenta is not accessible through a cervical approach, sampling in this twin pregnancy may be achieved using both cervical and abdominal approaches.
As with any invasive procedure, there are risks and benefits associated with chorionic villus sampling. Many patients opt for this test because it can be performed earlier than an amniocentesis. Reported complications of chorionic villus sampling include placental mosaicism, bleeding, infection, maternal–fetal hemorrhage, limb reduction defects, insufficient sample for analysis, and oromandibular hypogenesis.65–67 Moreover, any Rh-negative patient should receive Rhogam to prevent isoimmunization.
Second Trimester Screening
US/MRI: Ultrasound in discordant twins can define multiple anomalies, which may provide clues to chromosomal anomaly or genetic syndrome (Fig. 11.11A). MRI is sometimes utilized to exclude additional anomalies (Fig. 11.11B) and confirm diagnosis, particularly in the case of complicated monochorionic gestations (Fig. 11.12).

FIGURE 11.11: Dichorionic twin gestation with discordant anomalies. A: Ultrasound of discordant dichorionic twin gestation at 32 weeks showed cleft lip (arrow) and palate and complex cardiac anomalies (not shown) in one twin. B: SSFSE T2 sagittal imaging of the brain shows primary fissure (arrow) equally separating lobes of the vermis, consistent with small vermis inferiorly. The fetus also had micropthalmia. Postnatally, the twin was found to have trisomy 13.

FIGURE 11.12: Discordant Monochorionic Twins Axial SSFSE T2 imaging in an 18-week monochorionic gestation with twin-to-twin transfusion syndrome. One twin with alobar holoprosencephaly (arrow). Fetal MRI confirmed normal brain in the co-twin.
Biochemical Markers: In singletons, the second trimester quad test that combines maternal age with second trimester serum AFP, β-hCG, unconjugated estriol, and inhibin-A detects approximately 75% of cases of Down syndrome with a 5% false positive rate.68 Many medical centers do not routinely offer this quad screening to patients with multiple fetuses owing to the paucity of data related to the efficacy of second trimester maternal serum screening for aneuploidy in twins. Additionally, reports in the literature reflect the inconsistencies in both sensitivity and detection rates of these analytes and the difficultly in interpreting the variability. On average, maternal serum biochemical markers are twice as high in twins as in singletons of the same gestational age.69 Recently, Muller et al.70 evaluated second trimester maternal serum screening for Down syndrome in 3,292 twin pregnancies, and reported that median AFP levels were similar between dichorionic and monochorionic twins and that free β-hCG levels were higher in monochorionic pregnancies. Rates for Down syndrome detection and screen positivity, respectively, were 27.3% and 6.6% using maternal age alone, 54.5% and 24.6% using maternal age corrected for chorionicity, 54.5% and 7.75% using AFP and free β-hCG divided by 2, 54.5% and 8.05% using median values observed from the global twin population, and 54.5% and 7.75% using median values specific to monochorionic and dichorionic twins. The authors concluded that maternal serum screening is better than maternal age alone but still yields low detection rates. In another study71 of second trimester serum screening, high false positive rates led to an 18.3% amniocentesis rate for twins versus 7.5% rate in singletons. Thus, first trimester ultrasound screening has been suggested to be optimal for detection of Down syndrome.
Cell-Free Fetal DNA (cff DNA): In 1997, Dr. Lo and his coworkers discovered Cff DNA, an innovation that revolutionized prenatal screening. Cff DNA is released into the maternal bloodstream after placental cells undergo apoptosis.67 It constitutes approximately 10% of the total DNA in maternal plasma and is rapidly cleared from maternal blood, within 2 hours of delivery.72,73 The half-life of fetal DNA is short at approximately 16 minutes, but it has recently been found that the entire fetal genome, in the form of cell-free DNA, is present in maternal blood.74 Cell-free fetal DNA has been demonstrated as early as prior to the seventh week of gestation.75 Therefore, cff DNA has become the focus of research for the development of noninvasive prenatal testing (NIPT).
Of the several types, including digital polymerase chain reaction (PCR), massively parallel DNA sequencing (MPS) of the whole genome, and target sequencing of the selected genomic loci on the chromosome of interest, utilized for NIPT for detection of Down syndrome, the primary methods applied in the United States, Asia, and parts of Europe are MPS and target sequencing.76 The first approach, MPS, uses the whole genome and requires sequencing of many millions of DNA fragments to generate sufficient reads to detect differences in the level of chromosome 21, which constitutes 1.5% of sequenced fragments. The second alternative targets sequencing the select genomic loci on the chromosome of interest, for example, chromosome 21 for NIPT for Down syndrome.77–83 In a series of validation studies in singletons, the MPS technique demonstrated high accuracy with sensitivity rates between 79% and 100%, and <1% false positive rates for diagnosis of Down syndrome84–86; 97% to 100% for trisomy 1885–87; and 75% to 79% for trisomy 13.86,87 Of note, cell-free DNA should be timed appropriately, typically after the 9th week.
In twin pregnancies, recently, Huang et al.88 reported the use of Cff DNA in 189 twin pregnancies with sensitivity and specificity using maternal plasma sequencing for fetal trisomy 21 being both 100% and for fetal trisomy 18 at 50% and 100%, respectively. This study further supported that sequencing-based NIPT of trisomy 21 in twin pregnancies could be achieved with a high accuracy. However, additional studies are needed to validate NIPT in multiple gestations.
Karyotype/Invasive Procedures
Amniocentesis: Amniocentesis is the gold standard for prenatal diagnosis. The procedure is typically performed in the second trimester to obtain amniotic fluid for genetic analysis by a needle inserted through a prepped abdominal wall under continuous ultrasound guidance. Complications of amniocentesis include infection, bleeding, and maternal–fetal hemorrhage. As in chorionic villus sampling, an Rh negative patient should receive rhogam. Like most procedures, amniocentesis in twins presents unique diagnostic challenges. After correctly identifying placental location and the intended twin to be tested first, the needle is inserted into the amniotic sac of the first twin, followed by injection of indigo carmine before removing the needle. Methylene blue should not be used because of the risk of atresia of the fetal small bowel, staining of the skin, and methemoglobinemia in the infant. Next, the needle is inserted into the second twin’s amniotic sac. If the fluid aspirated appears blue tinged, the first sac is reentered and the physician should remove the needle and try again.89–91
ACOG reports the procedure-related loss rate from 1 per 300 to 500 performed.92 Several studies agree that the loss rate for twins exceeds this, but by what extent is controversial. Loss rates have approximated 1%93 in some studies, but others were inconclusive because of the heterogeneity of the populations studied and inconsistencies in the definition of procedure-related loss.49
Management
Management of the pregnancy is individualized and mainly expectant or termination. Selective termination is relatively safe in dichorionic twins but can be fraught with danger in monochorionic gestations, putting both twins at risk for demise.
Recurrence Risk
Recurrence risk is dependent on type of genetic transmission.
PRETERM DELIVERY
Definition/Incidence
Preterm delivery, defined as delivery before 37.0 weeks’ gestation, affects approximately 12% of singleton births in the United States1 and approximately 11% worldwide.94 Over half of twin deliveries occur before 37.0 weeks, and about one-tenth of twin deliveries before 32 weeks.1 Preterm delivery is the most significant cause of morbidity and mortality in twin gestations.
Pathogenesis
It is not surprising that multiple gestations are at risk for preterm labor given greater uterine distention, which can initiate labor and cause earlier cervical changes.
Diagnosis/Surveillance
Sonographic measurement of cervical length (Fig. 11.13) and biochemical testing of fetal fibronectin are the two main screening tools used to predict preterm birth.

FIGURE 11.13: Ultrasound of normal cervix with equal thickness of anterior and posterior cervical lips. X marks the internal cervical os; + marks the external cervical os; the echodense line connecting the two points is the endocervical canal.
Imaging
A shortened cervical length (Fig. 11.14) indicates a higher risk of preterm delivery for the twin pregnancy. Among 464 twin pregnancies seen for routine care, Skentou et al.95 found that the median cervical length at 23 weeks was 36 mm. The rate of spontaneous delivery before 33 weeks was inversely related to cervical length at 23 weeks. It increased gradually from approximately 2.5% at 60 mm to 5% at 40 mm, and to 12% at 25 mm, and exponentially below this length to 17% at 20 mm and to 80% at 8 mm (Fig. 11.15). A meta-analysis of studies that measured cervical length by transvaginal ultrasound to predict spontaneous preterm birth in twin pregnancies found that the test was most useful when performed in asymptomatic women at 20 to 24 weeks of gestation.96 In establishing a cutoff of 20 mm, the authors found that the positive likelihood ratios for preterm birth were 5.2 and 10.1, respectively, in the 35% of pregnancies delivered before 28 weeks and 39% before 32 weeks.96 The usefulness of screening cervical length is limited by high frequency of short cervices in women not at imminent risk of delivery and by the lack of effective interventions to prevent preterm birth. Consequently, universal screening in this population is controversial because there are no known treatments.

FIGURE 11.14: Shortened cervix by US and MRI. A: A transvaginal scan at 23 weeks in twin pregnancy shows dilatation and funneling of the internal cervical os (line with end arrows). The closed cervical length (dotted line) measures 2.5 cm with funneling cervix, with walls of funnel in a Y-shaped configuration. F is fetus. B: Transvaginal ultrasound with nearly completely effaced cervix, with U-shaped funnel. Same annotations in A. C: MRI imaging of U-shaped funneling and shortened cervical length. The black arrows show internal os, dotted lines show shortened closed cervix.

FIGURE 11.15: Risk of preterm delivery before 33 weeks’ gestation compared with closed cervical length at 23 weeks in twin pregnancies. The rate of spontaneous delivery before 33 weeks is inversely related to cervical length measured at 23 weeks. It increased gradually from approximately 2.5% at 6 cm to 5% at 4 cm, and 12% at 2.5 cm. (Adapted from Skentou C, Souka AP, To MSW, et al. Prediction of preterm delivery in twins by cervical assessment at 23 weeks. Ultrasound Obstet Gynecol. 2001;17:7–10.)
In contrast, for women with signs and symptoms of preterm labor between 23 and 33 weeks, cervical length was a better predictor of preterm delivery than funneling or digital examination.97 In symptomatic twin pregnancies, Crane et al.97 found rates of delivery were 44% for cervical lengths <15 mm as compared with no delivery within a week if cervical length was >25 mm.
Biochemical
Fetal fibronectin is a protein found between the fetal sac and the uterine lining that can be used to predict preterm birth in singleton and twin pregnancies. In the largest prospective study of 147 women expecting twins, fibronectin testing was done at 2-week intervals between weeks 24 and 30.98 Using fibronectin levels at 28 gestational weeks, Goldenberg et al.,98 reported deliveries prior to 32 weeks in 30% of women with a positive test (≥50 ng per mL) and 4% of women with a negative result. Although these data support the feasibility of fibronectin in predicting preterm twin birth, this testing is not routinely performed in asymptomatic twin pregnancies in the absence of other prematurity risk factors.
Management/Prevention
Progesterone has been the biggest advancement in the prevention of preterm birth in singleton pregnancies for women with a history of preterm delivery. However, this therapy does not extrapolate to twin gestations.99 Furthermore, in a recent meta-analysis, Sotiriadis et al.99 reported that progesterone use for prevention of preterm delivery in twin pregnancy was associated with increased rates of perinatal death. At this time, there are no effective methods of preventing preterm delivery in twins, even with a history of a preterm delivery.100
For women who have a short cervix, vaginal progesterone results are promising, but do not reach statistical significance in reducing preterm twin birth.101 Similarly, intramuscular injection of 17 hydroxyprogesterone does not reduce the risk for twin pregnancies complicated with a shortened cervix.102 Although cerclage has proven beneficial in preventing preterm birth in singleton gestations for women with a history of preterm delivery and short cervix, this benefit does not appear to be true for twin gestations. In fact, a 2005 meta-analysis showed a higher incidence of preterm birth in twin gestations with cerclage.103
Recently, there has been a report of cervical incompetence treated with a cerclage and pessary104 in a monochorionic monoamniotic twin pregnancy. Some authors have also proposed pessaries to prevent preterm birth in severe TTTS treated by laser.105 However, pessary use in preventing preterm birth in twins remains unclear.
Unfortunately, no intervention has been shown to improve outcomes in twins when the mother’s ultrasound has identified a short cervix or there is a history of preterm delivery.106 Since higher rates of preterm delivery occur in twins conceived by ART than those conceived naturally, the most effective strategy against morbidity and mortality associated with preterm delivery is the prevention of multiple gestations in the ART setting.
FETAL DEATH
Single intrauterine fetal death (sIUFD) in multiple gestation pregnancies occurs in roughly 3.7% to 6.8% of twin pregnancies.107–109 In 2006, a systematic review of 19 studies demonstrated that the increased risk of death of a co-surviving twin after the death of 1 twin was 12% for monochorionic pregnancies and 4% for dichorionic pregnancies. The odds ratio for monochorionic co-twin intrauterine death was six times that of dichorionic twins.110 Moreover, the studies reinforced that fetal death can occur at any time.
Single Twin Gestation Demise Before 14 Weeks (Vanishing Twin Syndrome)
This syndrome usually occurs in the first trimester with the identification of a single fetus weeks after confirmation and diagnosis of any twin pregnancy (Fig. 11.16). The true incidence of vanishing twins is unknown but may be as high as 29%.111 In vanishing twin syndrome, outcomes based on chorionicity are yet unknown. As for long-term outcome, Anand et al.112 reported no developmental delays in children up to age 1 year when comparing surviving twins from a vanishing twin pregnancy and children from singleton pregnancies.112

FIGURE 11.16: Vanishing twin syndrome in dichorionic pregnancy. A transvaginal scan at 9 weeks showing dichorionic twin pregnancy with thick separation (green arrow). Left sac is empty (representing vanishing twin), and right sac shows an embryonic pole.
Single Twin Demise After 14 Weeks
Incidence
The overall incidence of single twin death after 20 weeks of pregnancy is estimated between 2.6% and 6.2%.113
Pathogenesis
In primarily monochorionic twins, the morbidity and mortality in the surviving twin has been explained by two theories: transchorionic embolization and coagulopathy or transient hemodynamic fluctuations between the twins. In the first theory, passage of thrombotic materials from the dead twin occurs along the placental vascular anastomoses, resulting in disseminated intravascular coagulopathy in the initially healthy surviving twin.111 The resulting disseminated intravascular coagulopathy may result in infarcts and cystic degenerative changes in the survivor’s renal, pulmonary, hepatic, splenic, and neurologic organs.114 However, data have demonstrated normal coagulation in the surviving twin after intrauterine death of the co-twin, refuting this hypothesis.115
The alternative theory is based on rapid and profound hemodynamic alteration that occurs when one twin dies. Specifically, an acute shift of blood from the surviving twin flows into the dead fetus along the superficial anastomotic channels.115,116 There has been in utero demonstration of severe fetal anemia in the surviving twin after death of co-twin supporting this hypothesis.115,117
Etiology
The multiple causes for fetal death in twin pregnancy include fetal chromosomal or congenital malformations, and maternal diseases (e.g., diabetes, placenta diseases). Twin pregnancies complicated by a fetal death are at increased risk for both preterm delivery and death of the co-twin.
Diagnosis/Surveillance
Imaging: Regardless of chorionicity, management after a single fetal death is challenging. Expert opinions recommend ultrasound assessment of fetal growth every 2 to 4 weeks. In some cases of single fetal death in a multifetal pregnancy, prenatal ultrasound can detect intracranial abnormalities in the surviving twin.118 Patten et al.119 have demonstrated that a normal initial scan cannot rule out damage and that sonographic evidence of intracranial abnormalities in the surviving twin may manifest as early as 7 days after the death of its co-twin. Structural abnormalities observed in survivors include neural tube defects, optic nerve hypoplasia, hypoxic ischemic lesions of the white matter (multicystic encephalomalacia), microcephaly, hydranencephaly, porencephaly, hemorrhagic lesions (Fig. 11.17A), posthemorrhagic hydrocephalus, bilateral renal cortical necrosis, unilateral absence of a kidney, gastrointestinal tract atresia, gastroschisis, hemifacial microsomia, and aplasia cutis of the scalp, trunk, or limbs.120 Sonographic scan might be complemented with MRI.121 If time permits, the best timing for MRI is at 32 weeks or later, when white matter is developed and minor (yet clinically important) lesions in the white matter can be visualized. Some physicians advocate fetal MRI at least 3 weeks after single twin demise to assess brain injury in the surviving twin. Three patterns of brain pathology have been described in the surviving twin.111 First, hypoxic ischemic lesions of white matter usually occur in the area supplied by the middle cerebral artery, which may evolve to porencephaly, multicystic encephalomalacia, microcephaly, and hydrancephaly (Fig. 11.17B). Second, hemorrhagic lesions, either isolated or in combination with ischemic lesions, may lead to posthemorrhagic hydrocephalus (Fig. 11.17C). Third, anomalies secondary to a vascular disturbance can occur; these include neural tube defects, limb reduction anomalies, and optic nerve hypoplasia.

FIGURE 11.17: Twin Demise in Three Different Monochorionic Pregnancies with Intracranial Injury in the Surviving Twin. A: Axial cranial US of surviving twin after demise in a monochorioinc gestation demonstrates increased echogenicity in the ventricle, suggestive of hemorrhage (arrow). B: Coronal SSFP image in a monochorionic gestation at 21 weeks, 3 weeks after demise of co-twin shows moderate ventriculomegaly and areas of porencephaly/multicystic encephalomalacia (arrows) in the surviving twin. Fetus sent for MRI as US demonstrated increasing ventricular dilatation. C: Monochorionic twin gestation at 20 weeks with recent demise of co-twin (dotted arrows). The SSFSE T2 image demonstrates a large germinal matrix hemorrhage in the surviving twin (solid arrow).
Prognosis
Timing of fetal death and chorionicity are important factors that impact management, counseling, short-term, and long-term outcomes. In studies examining neurologic outcome after one fetal death in twin pregnancies,111 reports noted rates of cerebral palsy were 6.3% in surviving twins and 1.8% higher in monozygotic twin pregnancies. Nelson and Ellenberg122 also reported an increased rate of nonfebrile seizures in the surviving twin (5%) after fetal death versus 0.8% when both twins survive. In terms of IQ testing, no significant differences have been observed between survivors after their co-twin’s death and surviving twins.122
Management
Several factors impact management and outcome of the surviving co-twin. These include chorionicity, gestational age at time of fetal death, and maternal and placental diseases.
Monochorionic
Previable Pregnancy (<24 Weeks): Because of the lack of predictive investigation on long-term outcome at this gestational age, regardless of chronicity, most patients are managed conservatively during that period. Despite sparse data, some patients consider termination as another treatment option.123
Viable Pregnancy (≥24 Weeks): The prevalence of monochorionic twinning after a single fetal death ranges from 50% to 70%.124 Fetal death increases 1% to 2% per week in monochorionic gestations after 32 weeks.125 The surviving twin faces increased risk of fetal and neonatal morbidity and mortality, most often fetal death, neurologic morbidities, and prematurity. In a 2006 meta-analysis110 that assessed the risk of co-twin mortality and neurologic morbidity following the death of one twin after 14 weeks’ gestation, researchers reported a 12% risk of death and 18% neurologic abnormality for the monochorionic surviving twin.
Timing of Delivery: Based on the 2011 NICHD workshop, the proceedings recommended delivery if fetal death occurs at or after 34 weeks, and to consider delivery in cases before 34 weeks based on concurrent maternal and fetal conditions.125
Dichorionic: In the presence of single fetal death, the main risk in the surviving dichorionic diamniotic twin is preterm delivery. In 2006, a meta-analysis110 reported an estimated risk of both iatrogenic and spontaneous preterm delivery after single fetal death as being 68% and 57% in monochorionic and dichorionic pregnancies, respectively. Based on the NICHD workshop, management strategy for dichorionic diamniotic twin pregnancies is directed similarly to monochorionic diamniotic twin pregnancies.125 However, expert opinion recommends a conservative approach for dichorionic diamniotic twin pregnancies that includes regular fetal and maternal surveillance and delivery between 37 and 38 weeks.125 The mode of delivery should be based on the obstetrical indications for either vaginal birth or cesarean section. For maternal monitoring after fetal death, rhesus-negative women should undergo a Kleihauer test, and regular blood pressure monitoring because of the increased risk of preeclampsia.108
FETAL GROWTH ABNORMALITIES
Incidence
Patients with multifetal pregnancies are at risk for growth abnormalities, along with their associated increased risks of obstetric complications, and perinatal morbidity and mortality.121
Pathogenesis/Etiology
Growth discordance can occur secondary to multiple known risk factors classified as fetal (monochorionic, infections), placental (placenta previa, abruption, velamantous cord insertion), and maternal (maternal age >30 years, nulliparity, tobacco use, no prenatal care).126
Diagnosis/Surveillance
Normal Growth
The normal fetal growth trajectory of a twin gestation diverges around 8 weeks, slowing to a rate lower than for singletons.127–129 This rate persists until 30 to 32 weeks, at which time a second diversion in slower growth occurs. The slower growth rate in twins has been attributed to placental crowding and the more frequent anomalous umbilical cord insertion (Fig. 11.18). These findings have led to an emerging paradigm, explaining that events early in twin gestation, perhaps at the time of conception, play a critical role in determining intrauterine growth trajectories and size at birth. The American Congress of Obstetricians and Gynecologists (ACOG) technical bulletin on assessment of growth suggests that centers should use growth tables derived from twin gestations.127 However, most studies of twin growth curves are derived from a small sample size and do not take into account chorionicity, race, or sex, or represent singleton curves applied to multiple gestation.128,129

FIGURE 11.18: The 50th percentile of birth weight for gestational age was approximately similar among singletons, twins, and triplets before 28 weeks of gestation.
Growth Discordance:
In trying to improve the assessment of fetal weight in multiple gestations, several investigators have focused on individual biometric parameters. For example, an abdominal circumference ratio of <0.93 has a 3.8 likelihood ratio for twin 25% discordant weight, and a higher head circumference/abdominal circumference has increased rates of adverse pregnancy outcome. Another important assessment is growth discordance, with studies showing an association between significant differences in twin birth weights and increased mortality and morbidity.130–133
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree