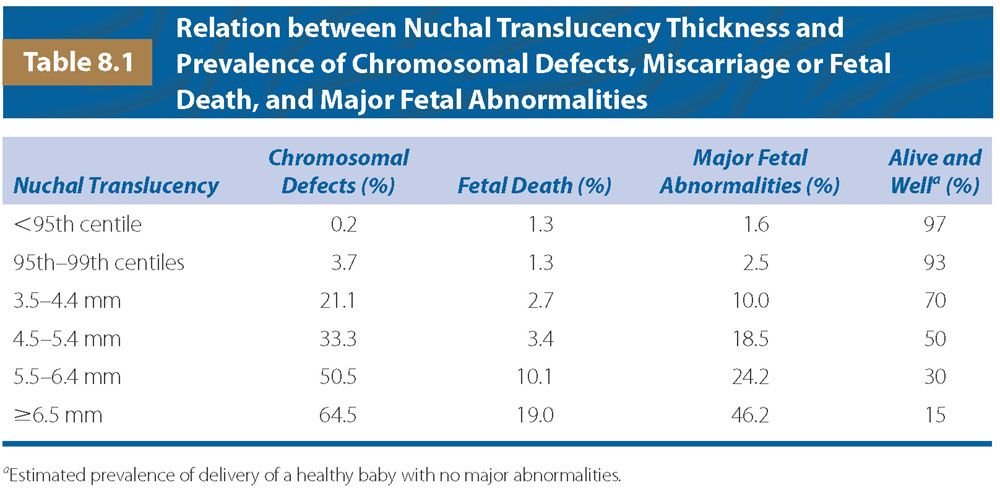
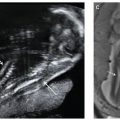
FIGURE 8.1: Ultrasound picture of a fetus at 12 weeks’ gestation illustrating the measurement of nuchal translucency (NT) thickness and assessment of the nasal bone (NB).
Implications of Increased Nuchal Translucency Thickness
The measurement of fetal NT thickness provides effective and early screening for trisomy 21 and other major aneuploidies.17–19 Furthermore, high NT is associated with fetal death, cardiac defects, and a wide range of other fetal malformations and genetic syndromes.20–24 The heterogeneity of conditions associated with increased NT suggests that there may not be a single underlying mechanism for the collection of fluid under the skin of the fetal neck. Possible mechanisms include cardiac dysfunction in association with abnormalities of the heart and great arteries, venous congestion in the head and neck, altered composition of the extracellular matrix, failure of lymphatic drainage due to abnormal or delayed development of the lymphatic system or impaired fetal movements, fetal anemia or hypoproteinemia, and congenital infection.
In normal fetuses NT thickness increases with fetal CRL. The median and 95th percentile of NT at a CRL of 45 mm are 1.2 and 2.1 mm, and the respective values at CRL of 84 mm are 1.9 and 2.7 mm. The 99th percentile does not change significantly with CRL, and it is about 3.5 mm. Increased NT refers to a measurement above the 95th percentile.
The prevalence of fetal abnormalities and adverse pregnancy outcome increases exponentially with NT thickness (Table 8.1). However, the parents can be reassured that the chances of delivering a baby with no major abnormalities are more than 90% if the fetal NT is between the 95th and 99th centiles, about 70% for NT of 3.5 to 4.4 mm, 50% for NT 4.5 to 5.4 mm, 30% for NT of 5.5 to 6.4 mm, and 15% for NT of 6.5 mm or more.
Management of Pregnancies with Increased Nuchal Translucency Thickness
In pregnancies with fetal NT below the 99th percentile (3.5 mm), the decision by the parents in favor of or against fetal karyotyping will depend on the patient-specific risk for chromosomal defects, which is derived from the combination of maternal age, sonographic findings, and serum-free β-hCG and PAPP-A. In terms of the subsequent management of the pregnancy, it would be best to carry out a detailed fetal scan at 20 weeks to determine fetal growth and diagnose or exclude major abnormalities that could not be identified at the 11 to 13+6 weeks scan.
A fetal NT above 3.5 mm is found in about 1% of pregnancies. The risk of major chromosomal abnormalities is very high and increases from about 20% for NT of 4.0 mm to 33% for NT of 5.0 mm, 50% for NT of 6.0 mm, and 65% for NT of 6.5 mm or more. Consequently, the first line of management of such pregnancies should be the offer of fetal karyotyping by CVS. In the chromosomally normal group, a detailed scan, including fetal echocardiography, should be attempted between 14 and 16 weeks to determine the evolution of the NT and to diagnose or exclude many fetal defects. If this scan demonstrates resolution of the NT with normal nuchal thickness measurement in the mid-trimester and absence of any major abnormalities, the parents can be reassured that the prognosis is likely to be good and the chances of delivering a baby with no major abnormalities is more than 95%. The only necessary additional investigation is a detailed scan at 20 to 22 weeks for the exclusion or diagnosis of both major abnormalities and the more subtle defects that are associated with certain associated genetic syndromes. If none of these is found, the parents can be counseled that the risk of delivering a baby with a serious abnormality or neurodevelopmental delay may not be higher than in the general population.
Persistence of unexplained increased NT at 14 to 16 weeks scan or evolution to nuchal edema or hydrops fetalis at 20 to 22 weeks raises the possibility of congenital infection or a genetic syndrome. Maternal blood should be tested for toxoplasmosis, cytomegalovirus, and parvovirus B19. Follow-up scans to define the evolution of the edema should be carried out every 4 weeks. Additionally, consideration should be given to DNA testing for certain genetic conditions, such as spinal muscular atrophy, even if there is no family history for these conditions. In pregnancies with unexplained nuchal edema at 20 to 22 weeks scan, the parents should be counseled that there is up to a 10% risk of evolution to hydrops and perinatal death or a live birth with a genetic syndrome, such as Noonan syndrome. The risk of neurodevelopmental delay is estimated at 3% to 5%.
Additional Ultrasound Markers
At 11 to 13 weeks absence of the fetal nasal bone, reversed a-wave in the ductus venosus, tricuspid regurgitation, and increased peak systolic velocity (PSV) in the hepatic artery are observed in about 60%, 66%, 55%, and 80% of fetuses with trisomy 21 and in 2.5%, 3.0%, 1.0%, and 5%, respectively, of euploid fetuses.25–34
Absent or Hypoplastic Nasal Bone
In the assessment of the nasal bone at 11 to 13 weeks scan, a midsagittal view of the fetal profile should be obtained, and the magnification of the image should be such that the head and upper thorax occupy the whole screen. The ultrasound transducer should be parallel to the direction of the nose, and the probe must be gently tilted from one side to the other of the fetal nose. The exact midsagittal plane of the fetal face is defined by the echogenic tip of the nose and rectangular shape of the palate anteriorly, the translucent diencephalon in the center, and the nuchal membrane posteriorly.
When these criteria are satisfied, it will be possible to visualize at the level of the fetal nose three distinct lines. Two of them, proximal to the forehead, will be horizontal and parallel to each other, resembling an “equal sign.” The top line represents the skin and the bottom one, usually thicker and more echogenic than the overlying skin, represents the nasal bone (see Fig. 8.1). The nasal bone is considered to be present if it is more echogenic than the overlying skin and absent if it is either not visible or its echogenicity is the same or less than that of the skin.
Abnormal Flow Across the Ductus Venosus
The ductus venosus is a short vessel connecting the umbilical vein to the inferior vena cava that plays a critical role in preferentially shunting oxygenated blood to the fetal brain. About 20% of oxygenated blood from the placenta bypasses the liver and is directed to the heart. It enters the right atrium and then is shunted across the foramen ovale into the left atrium. From the left atrium, the blood passes into the left ventricle and then the aorta. The ductus venosus usually closes within a few minutes after birth but this may take longer in preterm neonates.
Increased impedance to flow in the fetal ductus venosus at 11 to 13 weeks’ gestation is associated fetal aneuploidies, cardiac defects, and other adverse pregnancy outcomes.29,35–39 Blood flow in the ductus venosus has a characteristic waveform with high velocity during ventricular systole (S-wave) and diastole (D-wave) and forward flow during atrial contraction (a-wave). Most studies examining ductus venosus flow have classified the waveforms as normal, when the a-wave observed during atrial contraction is positive, or abnormal, when the a-wave is absent or reversed. The preferred alternative in the estimation of patient-specific risks for pregnancy complications is measurement of the pulsatility index for veins (PIV) as a continuous variable.40
In the assessment of ductus venosus flow, a right ventral midsagittal view of the fetal trunk should be obtained and the magnification of the image should be such that the fetal thorax and abdomen occupy the whole screen (Fig. 8.2). The examinations should be undertaken during fetal quiescence and color flow mapping must be used to demonstrate the umbilical vein, ductus venosus, and fetal heart. The pulsed Doppler sample should be small (0.5 to 1.0 mm) to avoid contamination from the adjacent veins and it must be placed in the yellowish aliasing area, the insonation angle should be less than 30°, the filter should be set at a low frequency (50 to 70 Hz) to allow visualization of the whole waveform and the sweep speed should be high (2 to 3 cm per second) so that the waveforms were widely spread. The ductus venosus PIV is measured by the machine after manual tracing of the outline of the waveform.
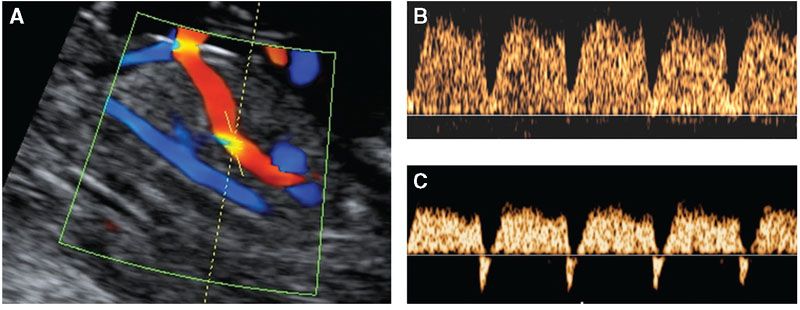
FIGURE 8.2: Midsagittal view of the fetal trunk demonstrating insonation of the ductus venosus (A) of a fetus at 12 weeks with normal waveform (B) and reversed a-wave (C).
Tricuspid Regurgitation
Tricuspid regurgitation at 11 to 13 weeks’ gestation is a common finding in fetal aneuploidies and major cardiac defects.30–32,41
In the assessment of tricuspid flow, an apical four-chamber view of the fetal heart should be obtained and the magnification of the image should be such that the fetal thorax occupies the whole screen. The pulsed Doppler sample should be large (2.0 to 3.0 mm) and positioned across the tricuspid valve, the insonation angle to the direction of flow should be less than 30° from the direction of the interventricular septum, and the sweep speed should be high (2 to 3 cm per second) so that the waveforms are widely spread (Fig. 8.3). The tricuspid valve could be insufficient in one or more of its three cusps, and therefore the sample volume should be placed across the valve at least three times, in an attempt to interrogate the complete valve. Tricuspid regurgitation is diagnosed, if it is found during at least half of the systole and with a velocity of over 60 cm per second, since aortic or pulmonary arterial blood flow at this gestation can produce a maximum velocity of 50 cm per second.
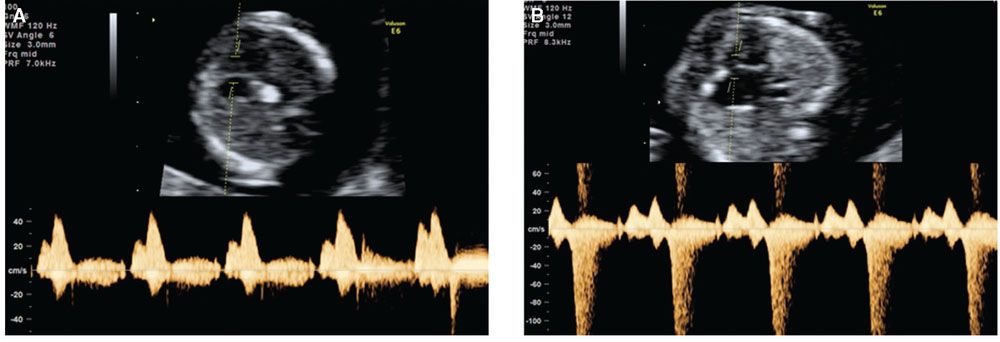
FIGURE 8.3: Transverse view of the fetal heart demonstrating Doppler assessment of flow across the tricuspid valve of a fetus at 12 weeks with normal waveform (A) and tricuspid regurgitation (B).
Increased Blood Velocity in the Hepatic Artery
In fetal life, the liver is a vital organ with both metabolic and hemopoietic activities. Normally, more than 90% of the blood supply to the liver is from the umbilical and portal veins and less than 10% comes directly from the hepatic artery that is a branch of the celiac trunk from the descending aorta. In trisomy 21 fetuses at 11 to 13 weeks’ gestation, the fetal hepatic artery PSV is increased and the PI is decreased.33,34
In the assessment of hepatic artery flow, a right ventral midsagittal view of the fetal trunk should be obtained and the magnification of the image should be such that the fetal thorax and abdomen occupy the whole screen. The examinations should be undertaken during fetal quiescence and color flow mapping should be used to demonstrate the umbilical vein, ductus venosus, descending aorta, and hepatic artery (Fig. 8.4). The pulsed Doppler sample must be set at 2.0 mm and placed so that it includes both the ductus venosus and the adjacent upper part of the hepatic artery (to ensure that this vessel rather than the celiac trunk is sampled) and it should then be reduced to 1.0 mm to include only the hepatic artery. The insonation angle to the hepatic artery must be less than 30°, the filter should be set at a high frequency (120 Hz) to avoid contamination from adjacent veins, the sweep speed should be high (2 to 3 cm per second) so that the waveforms are widely spread, and the pulsed wave pulse repetition frequency should be adjusted allowing better assessment of the PSV. When three similar consecutive waveforms are obtained, the PSV and PI are measured by the software of the machine after manual tracing.
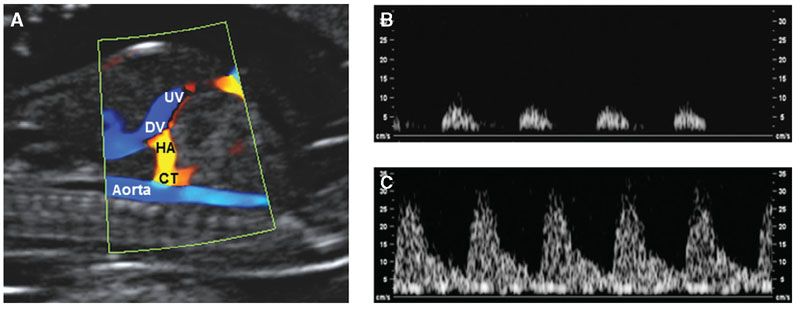
FIGURE 8.4: A: Right ventral midsagittal view of a fetus at 12 weeks demonstrating the umbilical vein (UV), ductus venosus (DV), descending aorta, hepatic artery (HA), and celiac trunk (CT). The peak systolic velocity in the waveform from the fetus with trisomy 21 (C) is much higher than in a euploid fetus (B).
Clinical Implementation
In first trimester combined screening, each of the additional ultrasound markers can be assessed in all patients resulting in an increase in DR from 93% to 96% and a decrease in FPR to less than 3%. A similar performance of screening can be achieved by a contingent policy in which first-stage screening by maternal age, fetal NT, and serum-free β-hCG and PAPP-A is offered to all cases (Fig. 8.5).42 Patients with a risk of 1 in 50 or more are considered to be screen positive and those with a risk of less than 1 in 1,000 are screen negative. Patients with the intermediate risk of 1 in 51 to 1 in 1,000, which constitutes 15% to 20% of the total population, have second-stage screening with nasal bone, ductus venosus, or tricuspid blood flow that modifies their first-stage risk. If the adjusted risk is 1 in 100 or more, the patients are considered to be screen positive and those with a risk of less than 1 in 100 is screen negative.
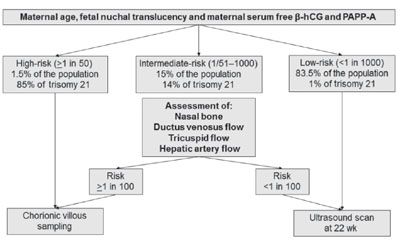
FIGURE 8.5: Two-stage screening for fetal aneuploidies. In the first stage, all patients have screening by a combination of maternal age, fetal nuchal translucency thickness, and maternal serum-free β-hCH and PAPP-A, and according to the results they are classified into high-risk, intermediate-risk, and low-risk groups. In the intermediate-risk group, second-stage screening is carried out by one or more sonographic markers, including nasal bone, blood flow in the ductus venosus, hepatic artery, or across the tricuspid valve, and on the basis of the results they are then classified as high-risk or low-risk groups.
Screening in Twins
The first step in screening for trisomies in twins is to determine chorionicity by ultrasound at 11 to 13 weeks (Fig. 8.6).43 In monochorionic twins, the average of the two NT measurements can be used to calculate the pregnancy risk and in dichorionic twins, the individual NT measurements can be used to calculate the fetus-specific risk.44,45 Measurement of serum-free β-hCG and PAPP-A is also useful in screening for trisomies in twins, but it is important to make the necessary adjustments because the levels change with gestation and they are lower in monochorionic than in dichorionic pregnancies.46–48
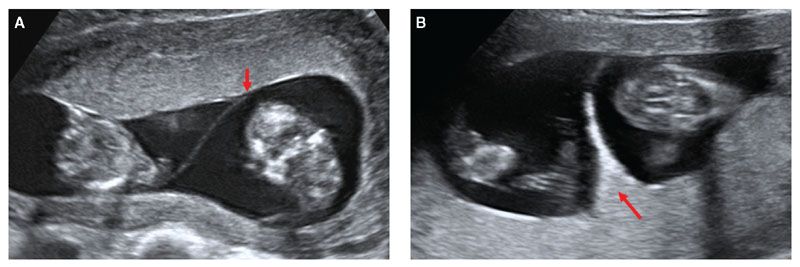
FIGURE 8.6: Ultrasound picture illustrating the difference in the junction of the intertwin membrane (shown by the red arrow) with the placenta in monochorionic (A) and dichorionic twins (B) at 12 weeks’ gestation.
In twins, the DR of trisomy 21 by the combined test is about 90% but at a higher FPR than in singletons (6% vs. 5%).44 In monochorionic twins, the FPR is even higher, at about 9%, because increased NT in one of the fetuses may be an early sign of twin-to-twin transfusion syndrome, rather than trisomy.44
Clinical Implementation of Cell-Free DNA Testing in Maternal Blood
The performance of screening for trisomies 21, 18, and 13 by cfDNA analysis of maternal blood is superior to that of the combined test.11 However, the test is expensive, and it is therefore unlikely that it would be used for routine screening of the whole population. We have suggested that the best model of screening is to offer cfDNA testing contingent on the results of first-line screening by the combined test.7,49 On the basis of the combined test, the population is divided into a very high-risk group, an intermediate-risk group, and a low-risk group. In this model, it is proposed that firstly, invasive testing is carried out in all cases in the very high-risk group, and secondly, cfDNA testing is carried out in the intermediate-risk group followed by invasive testing for those with a screen positive result (Fig. 8.7).
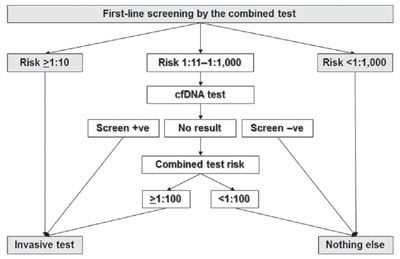
FIGURE 8.7: First-line screening by the combined test is carried out in all pregnancies. In those with a risk for trisomies 21, 18, or 13 ≥1:10, invasive testing is performed, and in those with a risk <1:1,000, there is no further testing. In women with risk between 1:11 and 1:1,000 cell-free (cf) DNA testing is carried out. In those with a positive cfDNA result, invasive testing is performed, and in those with a negative result, there is no further testing. In the group of women with no result from cfDNA testing, the results of the combined test are considered, and invasive testing is carried out for those with a risk for trisomy 21, 18, or 13 ≥1:100.
Such strategy would retain the advantages of the first trimester scan in the diagnosis of major defects and assessment of risk for pregnancy complications and would detect about 98% of fetuses with trisomies 21, 18, and 13, at an overall invasive testing rate of less than 1%. The intermediate-risk group requiring cfDNA testing constitutes about 25% of the population. However, this proportion can be reduced to about 10%, without affecting the overall performance of screening, by a first-line method of screening that includes measurement of ductus venosus PIV, in addition to fetal NT, FHR, and serum-free β-hCG, PAPP-A, PLGF, and AFP.49
DIAGNOSIS OF MAJOR FETAL DEFECTS
The 11 to 13 weeks scan evolved over the last 20 years from essentially a scan for the measurement of fetal NT and CRL to one that includes a basic checklist for examination of the fetal anatomy with the intention of diagnosing major abnormalities, which are either lethal or associated with severe handicap, so that the parents can have the option of earlier and safer pregnancy termination.
Major fetal abnormalities fall into essentially three groups in relation to whether they can be detected at the 11 to 13 weeks scan.50 Firstly, relatively easily detectable abnormalities, including body stalk anomaly, anencephaly, alobar holoprosencephaly, omphalocele, gastroschisis, and megacystis. The second category is anomalies not detectable in the first trimester, because they manifest only during the second or third trimester of pregnancy, including microcephaly, agenesis of the corpus callosum, semilobar holoprosencephaly, hypoplasia of the cerebellum or vermis, congenital pulmonary airway malformation, and bowel obstruction. A third group includes abnormalities that are potentially detectable in the first trimester, but whose diagnosis is significantly dependent on the objectives set for such a scan and consequently the time allocated for the fetal examination, the expertise of the sonographer, the quality of the equipment used and maternal body habitus, and secondly, the presence of an easily detectable marker for an underlying abnormality. A good example of such a marker in the first trimester is high NT that is found in some fetuses with lethal skeletal dysplasias, diaphragmatic hernia, and major cardiac defects.
Several studies reported the diagnosis of a wide range of fetal abnormalities during the first trimester scan. A randomized study of 35,792 pregnancies where a routine anomaly scan was carried out at 12 or 18 weeks using a checklist (skull, neck and brain, face, chest, heart, diaphragm, abdominal wall, stomach, kidneys, bladder, spine, and limbs), reported that the rate of prenatal detection of major abnormalities was not significantly different between the two groups (38% vs. 47%).51
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree