Fig. 5.1
Distribution of causes of sudden death in 1,435 young athletes (From Maron [55] with permission)
Normal Coronary Anatomy
A fundamental issue in the definition of coronary anomalies lies in the large spectrum of what is considered normal variation. It has been suggested that any morphologic form observed in >1 % of an unselected general population falls within the “normal variant” category [4]. An anomaly is an anatomic feature noted in <1 % of an unselected general population [5, 6].
Some researchers have suggested that there are two distinct parts of normal coronary circulation: the large, proximal epicardial vessels, and the distal microvascular, high-resistance vessels [7]. While the general assumption is that the human heart comprises of the right and left coronary arteries, the recommendation is to more accurately assume that the left anterior descending artery (LAD), left circumflex artery (LCX) and right coronary artery (RCA) are the fundamental units of coronary anatomy [7]. In this latter classification, the left main (LM) is considered a mixed proximal trunk that may or may not be present in all cases. The coronary orifices are located within the corresponding aortic sinuses; usually two, and occasionally three ostia are present. The conal branch of the RCA may arise separately from the right coronary sinus [8]. The LAD courses through the anterior interventricular groove while the LCX and RCA traverse the left and right atrioventricular grooves, respectively.
Embryology of Coronary Artery Development
Although the process of coronary embryogenesis is incompletely understood, it is postulated that the primitive endocardium makes a significant contribution to coronary endothelium while the vascular smooth muscle layer derives largely from the epicardium [9]. The process is believed to be mediated through complex signaling involving vascular endothelial growth factor (VEGF) which is elaborated by the myocardium. By the 25th day of embryonic development, a non-contiguous vascular plexus can be found surrounding the fetal heart (Fig. 5.2). At 6 weeks the vessels are continuous and connected to the aorta. Disruptions in this process are believed to underlie the development of coronary anomalies [10].
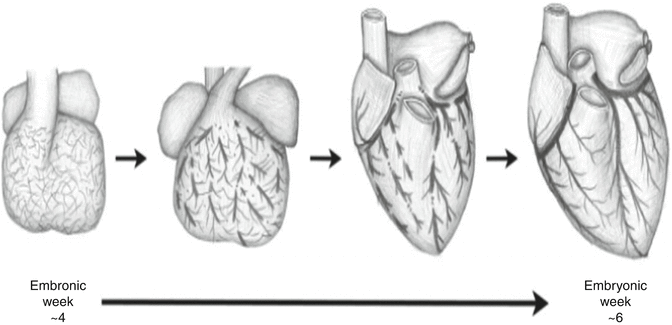

Fig. 5.2
Embryologic development of coronary vasculature (From Lluri and Aboulhosn [10] with permission)
Incidence
While the incidence of coronary anomalies is generally reported to be ~1 %, some series have reported a much higher incidence of ~5 % based upon a stricter classification scheme [7]. In one series of 126,595 invasive angiograms, the overall incidence was 1.33 % (Table 5.1) [11]. The rate of detection was similar in a coronary CT angiography (CTA) series [12].
Table 5.1
Incidence of coronary anomalies
Overall incidence (%) | % Frequency of all anomalies (%) | |
|---|---|---|
Probably benign | 1.07 | 80.6 |
Separate origin of LAD and Cx from LSV | 0.41 | 30.4 |
Cx from RSV or RCA | 0.37 | 27.7 |
RCA from the aorta | 0.15 | 11.2 |
Small coronary fistulae | 0.12 | 9.7 |
All other benign | 0.03 | 1.6 |
Potentially malignant | 0.26 | 19.4 |
Left main from the pulmonary artery | 0.008 | 0.059 |
LAD from the pulmonary artery | 0.0008 | 0.06 |
RCA from the pulmonary artery | 0.002 | 0.12 |
Left main from the right sinus | 0.017 | 1.3 |
LAD from the right sinus | 0.03 | 2.3 |
RCA from the left sinus | 0.107 | 8.1 |
Single coronary artery | 0.048 | 3.31 |
Large or multiple fistulae | 0.05 | 3.7 |
Classification of Anomalies
Many classification schemes have been proposed to describe the various configurations of coronary anomalies. The most thorough models describe three aspects of an anomalous artery; the origin, course and termination (Table 5.2) [1]. While this method is sure to describe any type of anomaly, in clinical practice it is more practical to think in terms of common anomaly types. One of the most common groups encountered by adult cardiologists is the anomalous coronary artery from the opposite sinus (ACAOS). These anomalies are further described by the course of the artery in relation to the great vessels, with an inter-arterial course (traversing between the aorta and pulmonary artery) to be the most concerning. Additionally, the finding of an intramural course (traversing within the aortic wall) is worrisome. Finally, coronary anomalies may either be benign or malignant in terms of prognosis, which lends the basis for another type of classification of coronary anomalies depending on the outcome [11]. The chapter will highlight the more common and/or significant in terms of coronary anomalies encountered in clinical practice.
Table 5.2
A comprehensive coronary artery anomaly classification scheme includes a description of origin, course and termination
A. Anomalies of origination and course |
1. Absent left main trunk (split origination of LCA) |
2. Anomalous location of coronary ostium within aortic root or near proper aortic sinus of Valsalva (for each artery) |
a. High |
b. Low |
c. Commissural |
3. Anomalous location of coronary ostium outside normal “coronary” aortic sinuses |
a. Right posterior aortic sinus |
b. Ascending aorta |
c. Left ventricle |
d. Right ventricle |
e. Pulmonary artery |
(1) LCA that arises from posterior facing sinus |
(2) Cx that arises from posterior facing sinus |
(3) LAD that arises from posterior facing sinus |
(4) RCA that arises from anterior right facing sinus |
(5) Ectopic location (outside facing sinuses) of any coronary artery from the pulmonary artery |
(a) From anterior left sinus |
(b) From pulmonary trunk |
(c) From pulmonary branch |
f. Aortic arch |
g. Innominate artery |
h. Right carotid artery |
i. Internal mammary artery |
j. Bronchial artery |
k. Subclavian artery |
l. Descending thoracic aorta |
4. Anomalous location of coronary ostium at improper sinus (which may involve joint origination or “single” coronary pattern) |
a. RCA that arises from left anterior sinus, with anomalous course |
(1) Posterior atrioventricular groove or retrocardiac |
(2) Retroaortic |
(3) Between aorta and pulmonary artery (intramural) |
(4) Intraseptal |
(5) Anterior to pulmonary outflow |
(6) Posteroanterior interventricular groove (wraparound) |
b. LAD that arises from right anterior sinus, with anomalous course |
(1) Between aorta and pulmonary artery (intramural) |
(2) Intraseptal |
(3) Anterior to pulmonary flow |
(4) Posteroanterior interventricular groove (wraparound) |
c. Cx that arises from right anterior sinus, with anomalous course |
(1) Posterior atrioventricular groove |
(2) Retroaortic |
d. LCA that arises from right anterior sinus, with anomalous course |
(1) Posterior atrioventricular groove |
(2) Retroaortic |
(3) Between aorta and pulmonary artery |
(4) Intraseptal |
(5) Anterior to pulmonary outflow |
(6) Posteroanterior interventricular groove |
5. Single coronary artery (see A4) |
B. Anomalies of intrinsic coronary arterial anatomy |
1. Congenital ostial stenosis or atresia (LCA, LAD, RCA, Cx) |
2. Coronary ostial dimple |
3. Coronary ectasia or anyeurism |
4. Absent coronary artery |
5. Coronary hypoplasia |
6. Intramural coronary artery (muscular bridge) |
7. Subendocardial coronary course |
8. Coronary crossing |
9. Anomalous origination of posterior descending artery from the anterior descending branch or a septal penetrating branch |
10. Split RCA |
a. Proximal + distal PDs that both arise from RCA |
b. Proximal PD that arises from RCA, distal PD that arises from LAD |
c. Parallel PDs ×2 (arising from RCA, Cx) or “codominant” |
11. Split LAD |
a. LAD = first large septal branch |
b. LAD, double (parallel LADs) |
12. Ectopic origination of first septal branch |
a. RCA |
b. Right sinus |
c. Diagonal |
d. Ramus |
e. Cx |
C. Anomalies of coronary termination |
1. Inadequate arteriolar/capillary ramifications |
2. Fistulas from RCA, LCA, or infundibular artery to: |
a. Right ventricle |
b. Right atrium |
c. Coronary sinus |
d. Superior vena cava |
e. Pulmonary artery |
f. Pulmonary vein |
g. Left atrium |
h. Left ventricle |
i. Multiple, right + left ventricles |
D. Anomalous anastomotic vessels |
Anomalous Coronary Artery from the Opposite Sinus (ACAOS)
In ACAOS (Fig. 5.3), the coronary arteries arise from the non-coronary sinus or the opposite coronary sinuses [13].
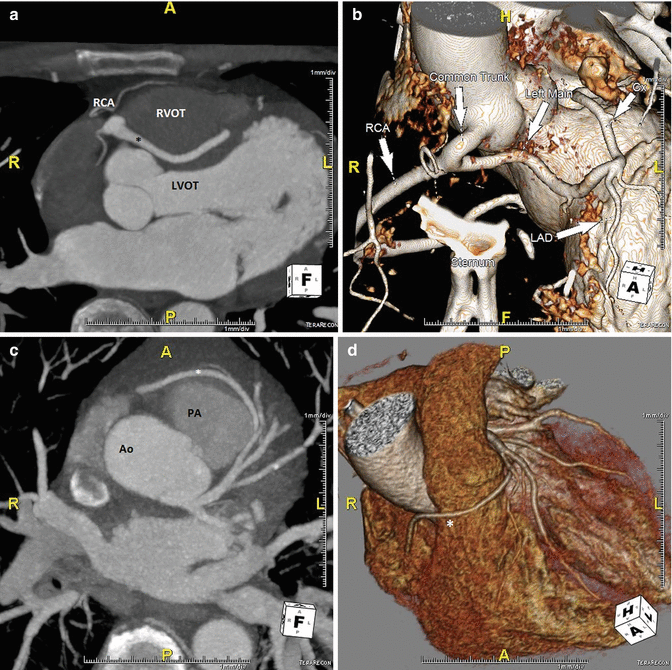
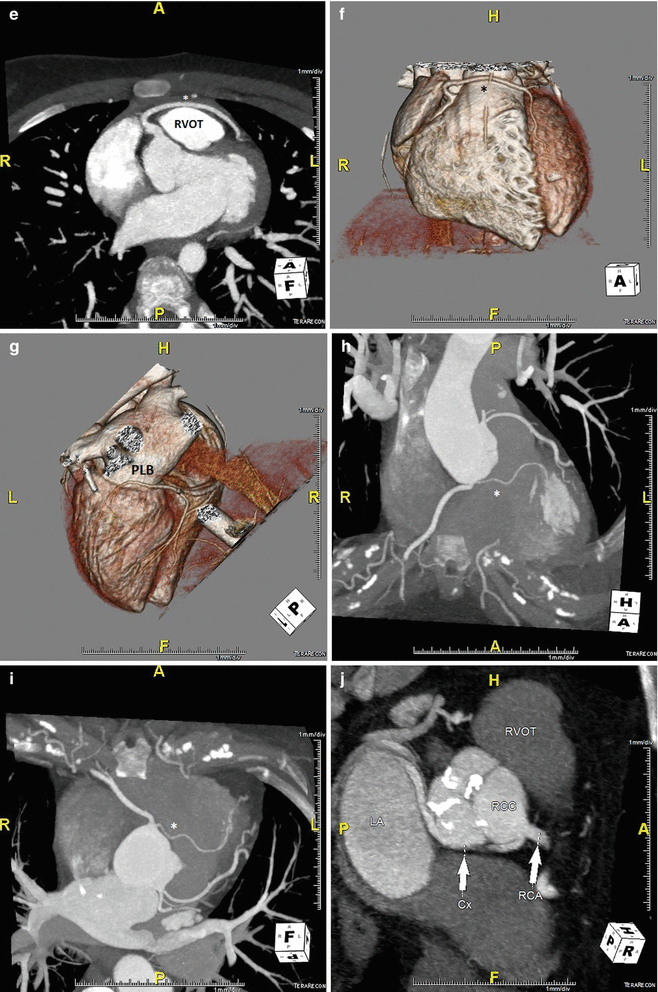
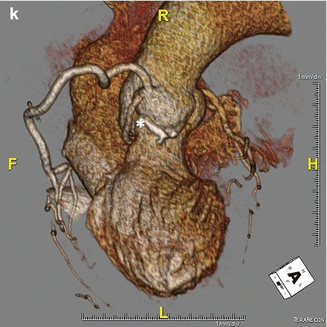



Fig. 5.3
(Panel a) An anomalous left main coronary artery arising from the right coronary artery; taking a low (inferior to the pulmonary valve) interarterial course (*). Typically, an anomalous left main coursing between the great arteries is recommended for surgical repair. A low interarterial course has been shown to be confer lower risk of sudden death when the anomalous vessel is a right coronary [11]. In this variant, the low course has implications for surgical management. (Panel b) A 3D rending of the anomaly from (panel a). The right ventricle and pulmonary artery have been removed. (Panels c, d). An anomalous right coronary artery arising from the left anterior descending taking a prepulmonic course (*). (Panels e, f, g) Tomographic and 3D renderings of an anomalous left anterior descending (*) arising from the right coronary artery, coursing anterior to the right ventricular outflow tract (RVOT). In (panel g) the right coronary artery is seen giving rise to a large posterolateral branch (PLB), owing to congenital absence of the left circumflex artery. (Panels h, i) An anomalous left coronary artery arising from the right sinus of Valsalva with a low interarterial course (*). The LAD is hypoplastic. Also seen in a large high obtuse marginal vessel which courses parallel to the LAD and supplies much of the territory commonly supplied by the LAD. (Panels j, k) Tomographic and 3D rendering of an anomalous left circumflex artery (*) arising from the right coronary cusp and coursing posterior to the aorta
1.
2.
The LM arises from the right coronary cusp in 0.09–0.11 %, and
3.
The LCX can arise from the right coronary cusp in 0.32–0.67 % of patients undergoing angiography [8].
Autopsy studies suggest up to a 57 % mortality rate for ACAOS involving an anomalous left coronary system and 25 % mortality rate for those involving the RCA [14]. There are limited natural history data available regarding the natural history of ACAOS. In one study, 24 of 72 middle-aged adults with ACAOS identified on coronary CTA, had an inter-arterial course. At a median follow-up of 15 months, two patients underwent corrective surgery and there was one death due to coronary disease (unrelated to the ACAOS). Additionally, 73 % of patients had improvement in the symptoms which led to the initial CTA while the other 27 % had worsening symptoms. Patients with anomalous RCA and inter-arterial compression had more frequent syncope and chest pain [15].
In a retrospective study of middle aged adults in Japan, 56 patients with ACAOS were followed for a median of 5.6 years. Of these patients, 78 % had anomalous RCA from the left coronary sinus with an inter-arterial course and none had an anomalous left main. All patients were managed with negative chronotropes and activity restriction despite symptoms attributable to ischemia and/or a positive stress test. During the study period, there was only a single cardiac death, attributed to aortic stenosis [16].
Anomalous Coronary Artery from the Pulmonary Artery, the Bland-White-Garland Syndrome
This syndrome (Fig. 5.4) constitutes the anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA). The identification of ALCAPA is most often encountered during the first weeks of life when pulmonary artery pressure falls to a range no longer able to support anterograde flow in the anomalous vessel. If sufficient collaterals are present, a coronary steal phenomenon may result. Occasionally, the pattern of collaterals may be sufficient to sustain life into adulthood. Over time, ventricular dilation, dysfunction and significant fibrosis develop.
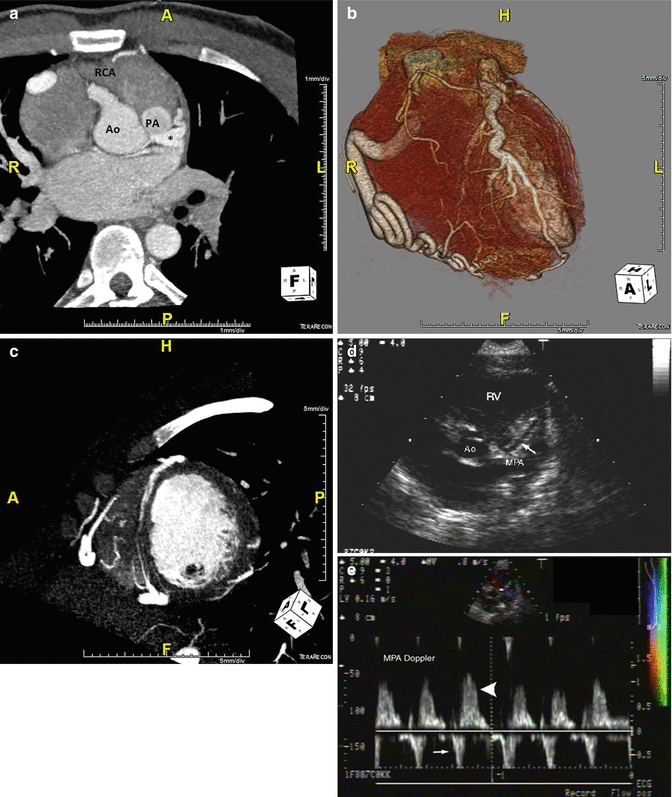

Fig. 5.4
A coronary CT angiogram from a 24 year old male with abdominal pain and easy fatigability. A CT. (Panel a) The coronary CT angiogram demonstrates an anomalous left coronary artery arising from the pulmonary trunk denoted with an asterisk as well as left heart enlargement. (Panel b) A 3D rendering demonstrating enlarged right and left coronary arteries, owing to a long standing high flow state. (Panel c) A short axis view showing robust epicardial and septal collateral network from the high-pressure right coronary artery through the left coronary artery and into the pulmonary artery. The patient underwent surgical reimplantation to the aorta with pericardial patch closure of the pulmonary trunk. (Panels d, e) Echocardiography demonstrating the LM coursing towards the pulmonary artery (white arrow) above the pulmonic valve (left). Doppler interrogation demonstrating abnormal diastolic flow within the main pulmonary artery towards the transducer, which represents runoff from the anomalous left coronary artery (large white arrowhead). There is normal systolic antegrade flow in the main pulmonary artery (small white arrow). Ao aorta, PA pulmonary artery, RCA right coronary artery (Panels d, e from Medscape Drugs & Diseases (http://emedicine.medscape.com/), 2015, available at: http://emedicine.medscape.com/article/893290-overview with permission)
Patients classically present in four ways; infant syndrome, mitral insufficiency, continuous murmur or sudden death. Infant syndrome represents 90 % of cases, with failure to thrive, angina-like episodes or congestive heart failure in the first several months of life. Mitral insufficiency tends to be functional and in the absence of structural valve disease, resolves with revascularization. A continuous murmur may be related to mitral insufficiency with excess diastolic mitral flow from the RCA to the anomalous left via collaterals [17].
Coronary Fistulas
A coronary fistula (Fig. 5.5) is an abnormal termination of a coronary artery into
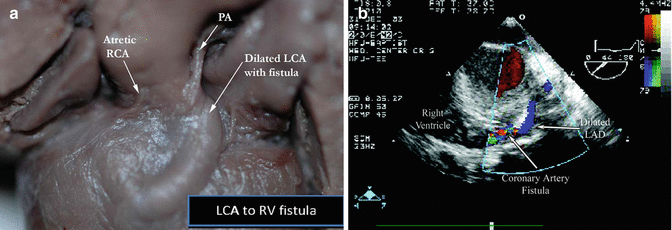

Fig. 5.5
A gross specimen (a) (left) of a fistula between the left coronary artery (LCA) to the right ventricle (RV), on the (b) right, echocardiographic demonstration of an LAD to RV fistula
1.
A cardiac chamber (coronary cameral fistula) or
2.
A low-pressure vessel as the pulmonary artery, pulmonary vein, and coronary sinus (coronary arteriovenous fistula).
Major sites of origin of fistula are the right coronary artery (55 %), left coronary artery (35 %), and both coronary arteries (5 %). Major termination sites are the right ventricle (40 %), right atrium (26 %), pulmonary arteries (17 %) and less frequently the superior vena cava or coronary sinus and least often the left atrium and left ventricle. This abnormality may lead to a coronary steal phenomenon, arrhythmia and volume overloading the chambers involved in the circuit. Presentation is typically due to incidentally discovered continuous murmur or symptoms of exertional dyspnea and fatigue. They are readily identified a coronary angiography, CT or MR but may be seen on echo, especially transesophageal echo. Unless they are small and asymptomatic, coronary fistula should be surgically treated since they rarely close on their own and frequently become symptomatic and the worse outcomes of repair with advancing age. Options include surgical ligation and if possible catheter embolization [17].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree