Chapter 13 IMAGING OF THE ANKLE
PATHOLOGY
Tendons
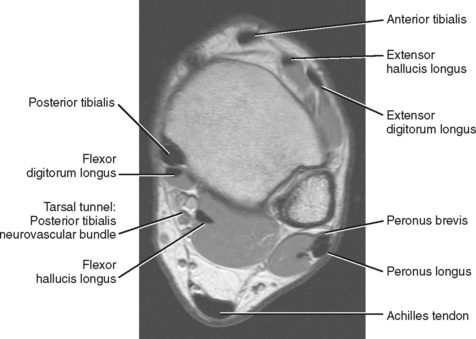
The tendons of the ankle can be divided into three compartments, anterior, lateral, and posterior, with the posterior compartment further subdivided into deep and superficial compartments (Fig. 13-1). A wide array of disorders can affect these tendons, including tenosynovitis, tendinopathy, tethering, subluxation or dislocation, partial and complete tears, tumors, ossification, and congenital abnormalities (Box 13-1). A thorough understanding of the normal anatomy and MR appearance along with knowledge of the common pitfalls is necessary to accurately evaluate the tendons of the ankle.
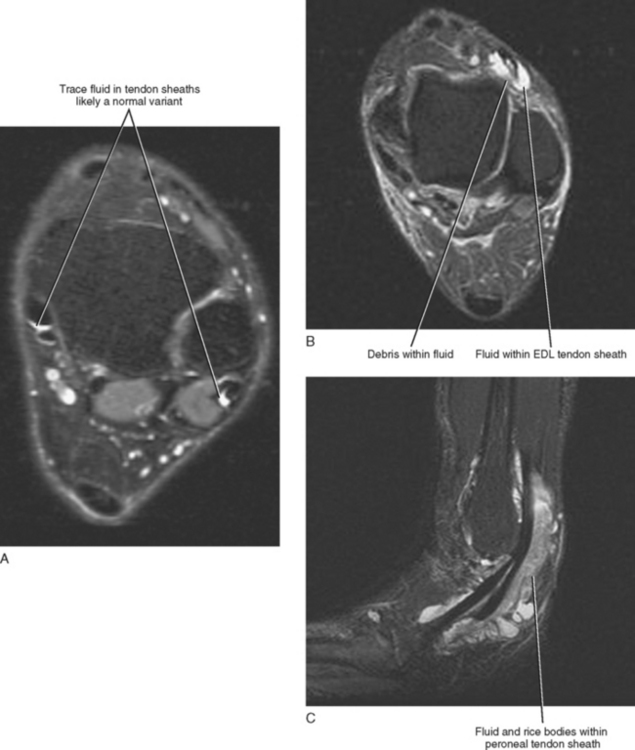
A small amount of fluid can occur within any of the tendon sheaths of the ankle in an asymptomatic patient, with the exception of the Achilles tendon, which does not have a surrounding sheath. Small asymptomatic fluid collections can be difficult to differentiate from symptomatic fluid because the precise volume of fluid indicative of disease has not been determined. A good rule of thumb to follow when evaluating the quantity of fluid within a tendon sheath is that any fluid collection smaller in diameter than the adjacent tendon is likely a normal physiologic finding and clinically insignificant, whereas a fluid collection that is equal to or greater in diameter than the adjacent tendon or a fluid collection containing complex debris (synechiae) is probably indicative of tenosynovitis (Fig. 13-2A). The one exception is the flexor hallucis longus (FHL) tendon sheath, which communicates freely with the ankle joint and can contain large quantities of fluid in asymptomatic patients. The diagnosis of FHL tenosynovitis should be considered when a fluid collection within the tendon sheath is large and out of proportion to the volume of the ankle effusion or when synechiae or debris are present.
Tenosynovitis usually results from repetitive overuse, but it may occur in association with an inflammatory arthropathy or may be infectious in origin. Differentiating between an infectious and an inflammatory tenosynovitis may not always be possible on the basis of MRI; however, infectious tenosynovitis often contains debris or appears complex and may demonstrate inflammatory changes, edema, and enhancement of the surrounding soft tissues. These signs, however, are not specific and can be seen with noninfectious tenosynovitis as well. Tenosynovitis associated with a chronic inflammatory arthropathy may contain rice bodies, which represent fibrinous exudative debris and often occur in conjunction with inflammatory changes of the adjacent joint, such as synovial thickening, erosions, and subchondral reactive marrow edema (Fig. 13-2C).
Several MRI pitfalls can potentially mimic tendon pathology (Box 13-2). Magic angle phenomenon is a common artifact resulting in increased signal within the substance of the tendon seen only on the T1-weighted images. It most commonly occurs in the posterior tibialis and peroneal tendons as they transition from the ankle into the foot, and it should not be misinterpreted as tendinosis. Phase-encoding artifact resulting from patient motion or from pulsation of blood within adjacent vessels can result in intrinsic signal within the substance of the tendon mimicking tendinosis. Finally, unfamiliarity with certain normal anatomic configurations or anatomic variations of the tendons can mimic disease. The posterior tibialis tendon normally broadens and splays and then splits just before its insertion on the tubercle of the navicular bone with the largest portion of the tendon inserting on the tubercle and several smaller slips extending into the midfoot to attach more distally. The appearance of the posterior tibialis tendon just proximal to its navicular insertion site can mimic focal tendinosis. With regard to normal anatomic structures mimicking pathology of the peroneal tendons, the presence of an accessory peroneus quartus tendon can mimic a split peroneus brevis tendon, and the calcaneofibular ligament, which is normally located just deep to the peroneal tendons below the distal tip of the fibula, can also mimic a split of the peroneus brevis tendon.
Anterior Compartment
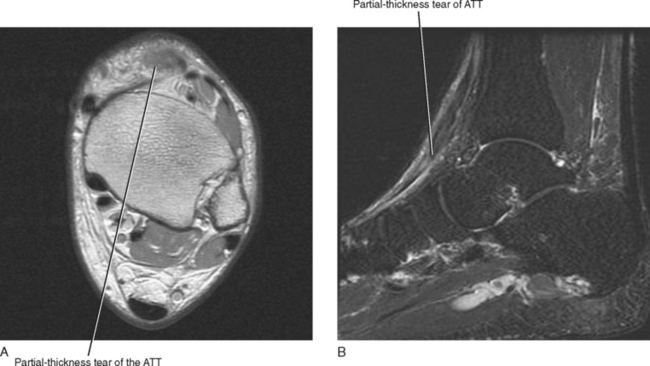
The tendons of the anterior compartment are responsible for dorsiflexion of the foot with the tibialis anterior tendon functioning as the primary dorsiflexor. From medial to lateral, the tendons include the anterior tibialis tendon, the extensor hallucis longus (EHL), and the extensor digitorum longus (EDL) tendons (see Fig. 13-1). Rupture of the anterior tibialis tendon is rare, occurring most commonly in people over 45-years of age and in athletes who participate in downhill running or marching and soccer kicking (Fig. 13-3). Injury also occasionally occurs as a result of direct trauma or laceration since the extensor tendons are superficial structures along the dorsal aspect of the foot and ankle. Unlike most tendons of the foot, which have dual blood supplies, the anterior tibialis tendon has a singular blood supply derived from the anterior tibial artery and as such is at increased risk for ischemia and injury in older persons with peripheral vascular disease. Ischemic changes of the anterior tibialis muscle and tendon classically manifest as a tender anterior soft tissue mass above the level of the ankle in the elderly patient with peripheral vascular disease.
It is less common to see physiologic fluid in the extensor compartment than in the flexor compartment tendon sheaths, and, as a result, any fluid—even a small quantity seen within an extensor tendon sheath—is more likely to be associated with symptoms (see Fig. 13-2A). Occasionally, an accessory tendon, the peroneus tertius, is present within the anterior compartment positioned lateral to the extensor digitorum tendon and should not be misinterpreted as a tear or split tendon. When present, the peroneus tertius tendon is seen as the “fourth extensor tendon” and is located lateral to the extensor digitorum longus tendon and inserts on the base of the fifth metatarsal.
Posterior Compartment
The tendons within the deep aspect of the posterior compartment are primarily responsible for plantar flexion and inversion of the foot. From medial to lateral are the posterior tibialis tendon, the flexor digitorum longus (FDL) and the flexor hallucis longus (FHL) tendons (see Fig. 13-1). The tarsal tunnel is a confined space along the posterior medial aspect of the ankle that is bound superficially by the flexor retinaculum and deep by the posterior border of the tibia and talus. It contains the posterior tibialis tendon, FDL and FHL tendons and the posterior tibialis neurovascular bundle.
Posterior Tibialis Tendon
The posterior tibialis (PT) tendon is the primary inverter of the foot and also provides important stability to the arch of the midfoot. Posterior tibialis tendon dysfunction refers to a spectrum of abnormalities ranging from mild tendinosis to complete tendon rupture resulting in medial sided ankle pain. Although complete rupture is rare, it is a devastating injury that leads to progressive collapse of the arch of the foot and a painful degenerative midfoot arthritis that often requires a triple arthrodesis for stabilization and pain relief. Posterior tibialis tendon dysfunction most commonly occurs in women over age 50, but other predisposing factors include prior flatfoot deformities, diabetes, renal failure, rheumatoid arthritis, and seronegative arthropathies. Acute disruption can also occur in young athletes participating in sports that require rapid change of direction, and it has been reported in ballet dancers and soccer and basketball players.
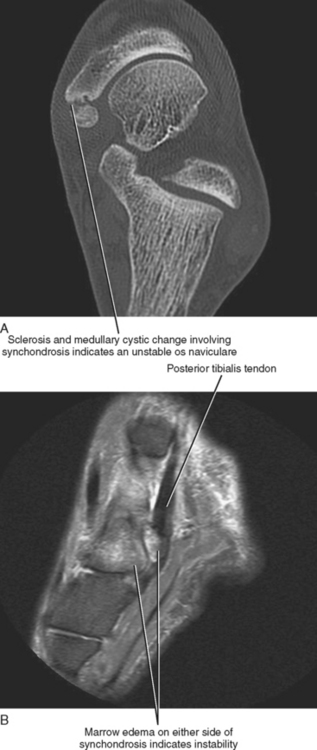
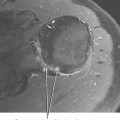
The os naviculare is a common normal variant accessory ossification center located at the level of the navicular tubercle. Os naviculare can be classified as type 1—small accessory ossicle contained with the posterior tibialis tendon with no articulation to the navicular; type 2—large accessory ossicle with an articular facet (synchondrosis); and type 3—a cornuate bony navicular tuberosity. Types 2 and 3 are often associated with an increased incidence of posterior tibialis tendon dysfunction and medial-sided ankle pain. MRI signs that are associated with a symptomatic os naviculare include marrow edema within the accessory ossification center and adjacent soft tissue edema. An unstable synchondrosis can also be associated with medial-sided ankle pain. The presence of fluid within the synchondrosis, subcortical cysts, sclerosis, and marrow edema on either side of the synchondrosis are MR and CT imaging signs, thus suggesting instability of the os naviculare, and indicate a potential unstable attachment of the posterior tibialis tendon (Fig. 13-4).
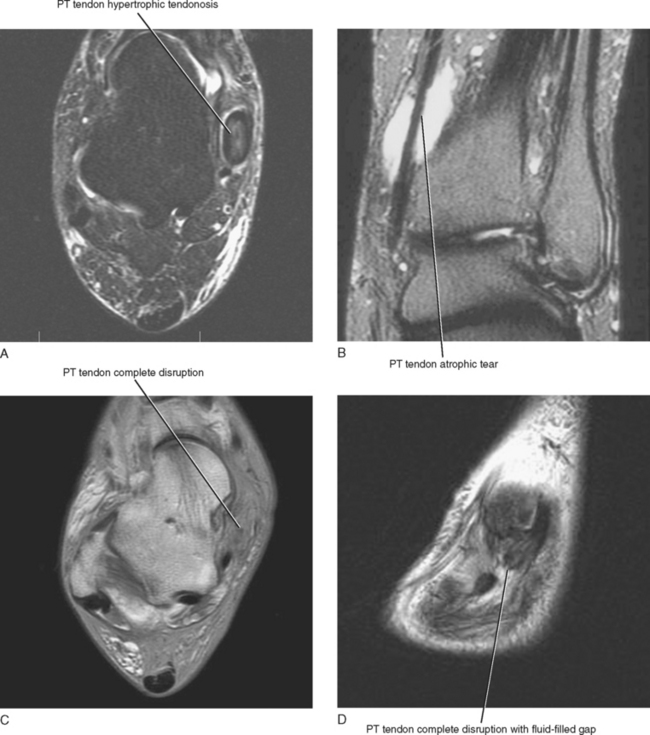
An MRI grading system has been described, which can be used to quantify abnormalities of the posterior tibialis tendon. The various grades have surgical implications (Table 13-1). A type I tear is seen as thickening of the tendon with intrinsic signal alteration and is referred to as hypertrophic tendinosis (Fig. 13-5). Partial-thickness interstitial longitudinal tears often accompany hypertrophic tendinosis and appear on MRI as fluid signal intensity streaks within the substance of the tendon. A type I tear is usually treated conservatively. A type II tear is considered atrophic with thinning and attenuation of the tendon. The tendon is usually thinner than the adjacent FDL tendon, and this type of tear typically requires surgical repair. A type III tear is complete seen on MRI as disruption of the tendon with a fluid-filled gap and retraction of the torn tendon ends. Types II and III tears lead to progressive midfoot collapse and osteoarthritis, resulting in chronic midfoot pain.
Table 13-1 Posterior Tibialis Tendon Dysfunction: MRI Grading System
| Type | Description of Tendon | Treatment |
|---|---|---|
| I | Hypertrophic (thickened) | Conservative |
| II | Atrophic (thinned/attenuated) | Surgical |
| III | Complete tear | Surgical |
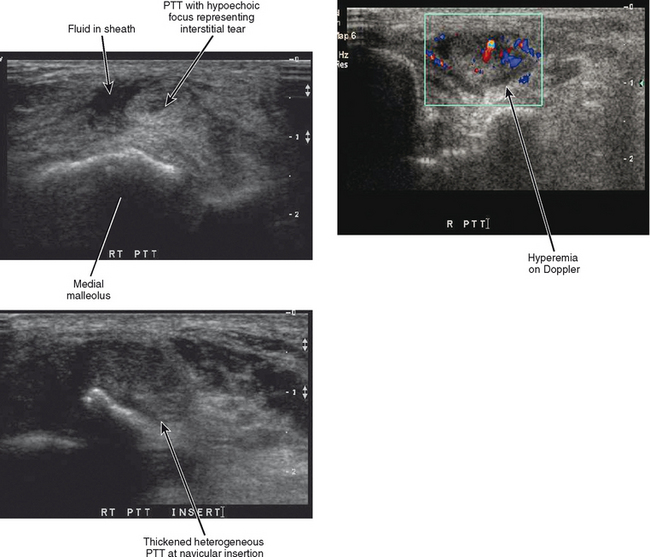
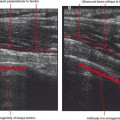
A four-point classification system is used for clinical staging of posterior tibialis dysfunction (Table 13-2), which deals more with the clinical presentation than with the extent of posterior tibialis tendon pathology. Stage I is tenosynovitis in which the patient presents with medial ankle pain and swelling. At this stage, the tendon remains normal and there is no arthritis of the midfoot. Stage II is a tendinopathy with flexibility of the ankle and midfoot. There may be early arthritis of the midfoot, but the midfoot remains flexible and the deformity is passively correctible. Stage III is tendinosis with fixed arthritic changes of the midfoot. At this stage, the deformity of the midfoot is no longer passively correctible. This stage of disease is most commonly seen in patients over 60 years of age. Stage IV is tendinopathy with rigid arthritis of the midfoot and ankle. At this stage, radiographs or MRI demonstrate arthritis of the tibiotalar joint and ankle valgus in addition to the arthritic changes of the midfoot. Surgical treatment of stage IV disease usually consists of triple arthrodesis, possibly with deltoid repair or calcaneal osteotomy. Ultrasound examination with power Doppler can also accurately detect and classify abnormalities of the posterior tibialis tendon (Fig. 13-6).
Table 13-2 Posterior Tibialis Tendon Dysfunction: Surgical Grading System
| Stage | Clinical Findings | Treatment Options |
|---|---|---|
| I | Tendinosis/tenosynovitis | Conservative |
| II | Tendinopathy/flexible | Brace: Low risk, lifelong, does not correct deformity |
| Correctible | Surgery: Corrects deformity, long recovery | |
| III | Fixed/midfoot arthritis | Brace |
| Surgery: Triple arthrodesis | ||
| IV | Fixed/midfoot and ankle arthritis/ankle varus deformity | Surgery: Triple arthrodesis with calcaneal osteotomy; triple arthrodesis with deltoid repair |
Flexor Digitorum Longus Tendon
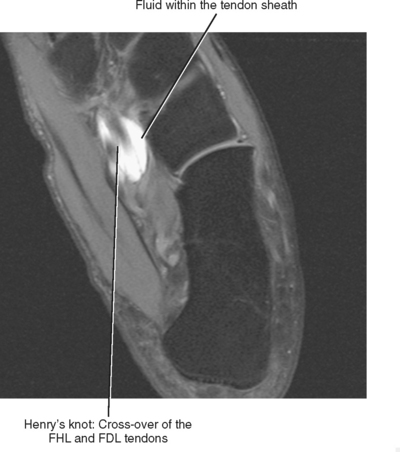
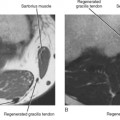
At the level of the ankle, the flexor digitorum longus (FDL) tendon is positioned between the posterior tibialis tendon and the flexor hallucis longus (FHL) tendon (see Fig. 13-1). As the FDL tendon extends into the midfoot, it crosses superficial to the FHL tendon (an anatomic landmark called Henry’s knot) and then gives off a tendon slip to each of the second through fifth digits. Isolated abnormalities of the FDL tendon are rare, but it is not uncommon to have tenosynovitis of the FDL tendon sheath in conjunction with tenosynovitis of the adjacent posterior tibialis or FHL tendons. Tenosynovitis of the FDL tendon is most likely to occur in the midfoot at the level of intersection with the FHL tendon, and MRI will demonstrate the presence of fluid within the tendon sheath at the level of Henry’s knot (Fig. 13-7).
Flexor Hallucis Longus Tendon
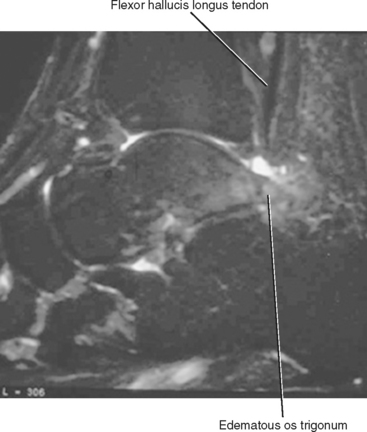
Tendinosis of the FHL most commonly occurs in athletes who perform extreme plantar flexion and push-off from a plantar-flexed position. It can also occur in association with an os trigonum, or an unfused posterior lateral tubercle of the talus, which has been referred to as the os trigonum syndrome (Fig. 13-8). Tendinosis of the FHL most commonly occurs at the level of the tibiotalar joint, but another common location includes the midfoot at Henry’s knot where the FHL and FDL tendons intersect.
Achilles Tendon
The Achilles tendon is the most superficial of the flexor tendons at the level of the ankle and is formed by a confluence of fibers arising from the soleus and the gastrocnemius muscles and attaches distally to the posterior calcaneus. The Achilles tendon differs from most tendons in that it lacks a sheath and is instead covered by a thin membrane and a delicate network of blood vessels referred to as a paratenon (see Fig. 13-1). Injury is usually secondary to chronic overuse and is most prevalent in middle-aged men. Achilles tendinosis can also be associated with chronic steroid use and numerous systemic diseases such as rheumatoid arthritis, diabetes, gout, chronic renal failure, collagen vascular disease, and fluoroquinolone therapy.
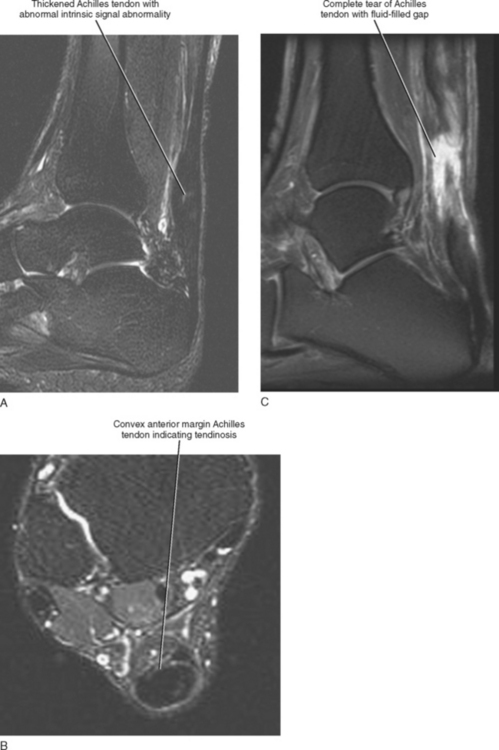
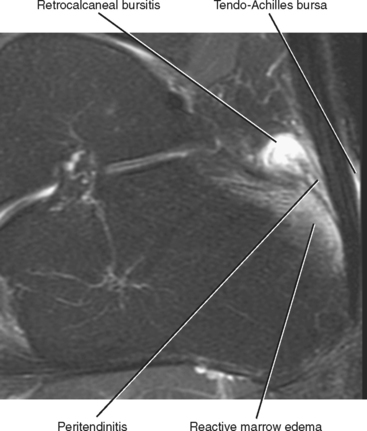
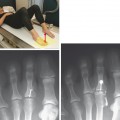
Injuries of the Achilles tendon are classified as either insertional or noninsertional. The noninsertional injuries most often occur at the watershed vascular zone approximately 4 to 6 cm above the distal insertion or at the musculotendinous junction. Noninsertional injuries also include proximal myotendinous junction strains of the Achilles tendon. These injuries can be classified as peritendinitis, tendinosis, or partial- or full-thickness tear (Fig. 13-9). Peritendinitis is the mildest form of injury, and on MRI, the Achilles tendon is normal in appearance; however, fluid and edema can be seen surrounding the tendon and within the pre-Achilles fat (Kager’s fat pad). A small fluid collection may also be present within the retrocalaneal bursa.
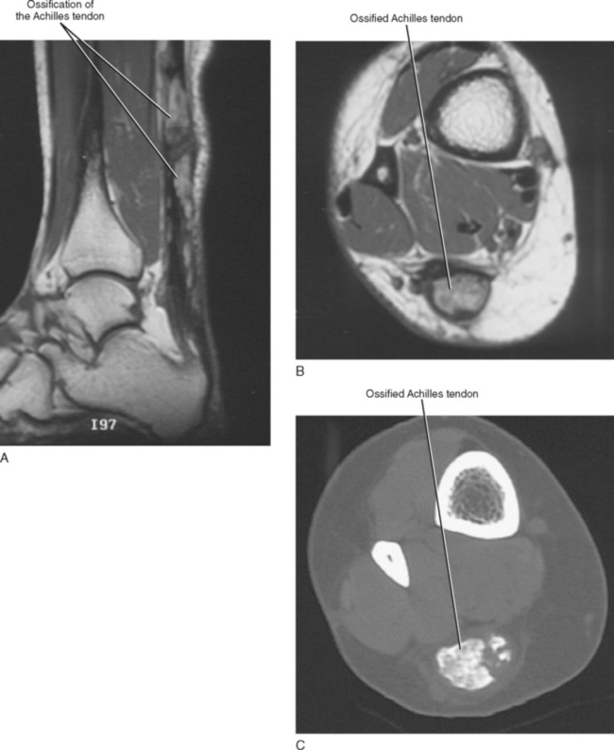
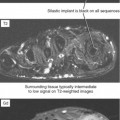
Tendinosis is usually seen as thickening of the tendon and may also demonstrate abnormal intrinsic signal representing intrasubstance myxoid degeneration. Sagittal MRI shows fusiform thickening of the tendon, and axial images demonstrate a rounded or convex anterior margin. Hypoxic tendinosis shows low signal on both T1- and T2-weighted images and thickening of the Achilles tendon. Calcific tendinosis is a rather common finding associated with chronic Achilles tendinosis. Ossification of the Achilles tendon, on the other hand, is an uncommon complication that has been reported after trauma to the Achilles tendon, resulting in either a partial- or full-thickness tear of the tendon or in surgery (Fig. 13-10). Calcific tendinosis may be difficult to detect on MRI but is easily seen on radiographs of the ankle. Ossification of the Achilles tendon demonstrates as areas of high T1-weighted signal representing areas of fat within mature marrow surrounded by dark-rimmed cortex within the substance of a thickened Achilles tendon. Ossification at the level of distal insertion of the Achilles tendon usually represents enthesopathy and is of no clinical significance.
Fluid signal within the substance of the tendon indicates a partial-thickness tear. This is often seen initially as linear longitudinal streaks of fluid signal indicating longitudinal interstitial tearing. Intermediate signal streaks, however, within the substance of the tendon distally can be a normal finding. A high-grade partial-thickness tear may result in marked thinning and attenuation of the tendon and in partial retraction of the torn portion of the tendon. A full-thickness tear demonstrates complete discontinuity of the fibers with a fluid-filled gap and retraction of the torn tendon ends. A complete description should include the location of the tear as it relates to the level of distal attachment, the extent of retraction of the proximal tendon end, and the length of the gap between the torn tendon ends. The status of the torn tendon end is also important for presurgical planning, and a complete description should indicate the presence of thickening, edema, fraying, or irregularity of the torn tendon ends. A description of an intact plantaris tendon should also be described because this can lead to a false-negative clinical exam in the presence of a complete Achilles tendon tear.
Insertional Achilles tendon abnormalities occur at the distal attachment site and are often associated with enthesiophyte formation and calcification within the distal tendon. Insertional abnormalities include tendinosis and partial- and full-thickness tear. Inflammatory changes and fluid are often present within the superficial adventitial (retro-Achilles) bursa as well as within the deep retrocalcaneal bursa (Fig. 13-11). Subcortical marrow edema is commonly seen in the posterior calcaneal tubercle in association with insertional Achilles tendinosis. Marrow signal in this situation is usually reactive in etiology and should not be mistaken for osteomyelitis.
Haglund syndrome describes a specific type of insertional Achilles tendinosis that is associated with a bony prominence (a Haglund deformity) extending off of the superior aspect of the posterior calcaneus, which results in impingement of the deep fibers of the Achilles tendon just above the level of distal attachment (Fig. 13-12). This may be exacerbated by certain footwear, especially pumps (e.g., “pump bump”). Repetitive impingement results in fraying and irregularity of the deep fibers of the Achilles tendon and eventually leads to a partial- or full-thickness tear. Treatment includes not only debridement or repair of the tendon abnormality but also an osteotomy of the bony prominence to prevent recurrent impingement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree