WHAT IS NUCLEAR MEDICINE?
Nuclear medicine uses radioactive compounds called radiopharmaceuticals or radiotracers that interrogate physiologic or pathologic processes at a molecular level and provide targeted therapy for a variety of diseases. Nuclear medicine studies are clinically used to assess most organ systems with almost 100 different types of studies or therapies performed in the United States. Many more radiotracers play a crucial role in research.
When used as an imaging agent to evaluate organ function, metabolism, or membrane receptor characteristics, the amount of radiotracer administered is in the picomolar or nanomolar range, which avoids disturbing the process under evaluation while still yielding data that are quantifiable and comparable to normative standards. As functional deficits arise before morphological changes in many diseases, nuclear medicine studies can detect disease in early stages when curative or more effective palliative treatment choices may be an option. This chapter reviews the fundamentals of imaging generation for gamma and positron-emitting radiopharmaceuticals and common nuclear medicine procedures based on the organ system.
IMAGE GENERATION
Radiopharmaceuticals contain radioactive atoms that are unstable and produce ionizing radiation when they decay. High-energy rays produced by the decay interact with special light-producing crystals in nuclear medicine cameras to create the images. The types of imaging rays from radioisotope decay can be broadly placed in two categories: gamma rays or positron emission.
When gamma ray emitters such as technetium-99m decay, rays are emitted of a specific energy typically reported in kiloelectron volts (keV). For example, the workhorse of gamma imaging, technetium-99m, decays to produce a single 140 keV ray for imaging. Some radioisotopes give off several imaging rays, and gamma cameras are able to distinguish between the specific energy rays allowing for simultaneous imaging of two or more radiotracers.
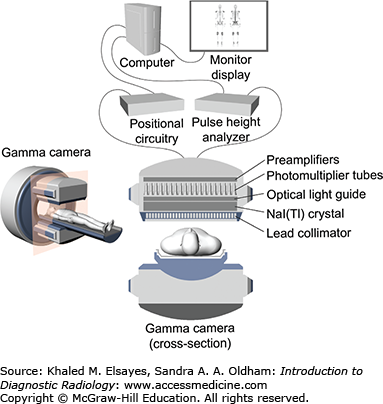
Gamma cameras fundamentally are composed of 4 main parts: the collimator, a crystal, photomultiplier tubes, and electronics for processing the data (Fig. 2.1). A collimator is a sheet of lead with holes designed to reduce scatter when placed between the patient and the crystal. After interacting with the gamma rays that passed through the collimator, the sodium iodide doped with thallium crystal NaI(Tl) emits faint light, which is then detected by the photomultiplier tubes and processed into viewable nuclear images or scintigrams. The two-dimensional images obtained are termed “planar” (Fig. 2.2). Because the gamma rays are coming from within the patient as opposed to from an external source as in conventional imaging, the change in the position of the camera alters the image dramatically as the tissue attenuation changes. Figure 2.3 depicts the steps necessary to obtain images of a patient injected with a radiopharmaceutical intravenously. Imaging protocols vary depending on the type of radiopharmaceutical utilized, route of administration, and clinical question.
Fig. 2.3
Serial photographs, from left to right, illustrate the steps necessary to obtain images of a patient injected intravenously with a radiopharmaceutical. The syringe containing the radiopharmaceutical is provided from the radiopharmacy in a lead-shielded container called a unit dose pig. After the radiotracer is injected intravenously, the patient lies on a table under the gamma camera and images are obtained according to specific protocols.
When the gamma camera is rotated around the patient, a three-dimensional data set is collected in a process termed single photon emission computed tomography (SPECT) and displayed in a multiplanar format—axial, coronal, and sagittal (Fig. 2.4). SPECT images allow for better localization of findings on planar images and can aid in the visualization of lesions obscured by overlying tracer activity just as chest CT can help evaluate a lung nodule seen on a chest radiograph. Increasingly, gamma cameras are equipped with an integrated CT scanner allowing for SPECT-CT studies. The merging of functional and anatomic data allows precise localization and appears to improve study interpretation, although the literature currently has few studies to support this. Table 2.1 lists commonly used single photon radionuclides in nuclear medicine and their physical characteristics.
Commonly Used Single-Photon Radionuclides and Their Physical Characteristics
| Radionuclide | Principal Mode of Decay | Physical Half-Life | Principal Photon Energy (keV) and Abundance | Production Method |
|---|---|---|---|---|
| 67Gallium | Electron capture | 78.3 h | 93 (37%) | Cyclotron |
| 185 (20%) | ||||
| 300 (17%) | ||||
| 395 (5%) | ||||
| 99Molybdenum | Beta minus | 2.8 d | 740 (12%) | Reactor |
| 780 (4%) | ||||
| 99mTechnetium | Isomeric transition | 6 h | 140 (89%) | Generator (99Molybdenum) |
| 111Indium | Electron capture | 2.8 d | 171 (90%) | Cyclotron |
| 245 (94%) | ||||
| 123Iodine | Electron capture | 13.2 h | 159 (83%) | Cyclotron |
| 131Iodine | Beta minus | 8 d | 364 (81%) | Reactor |
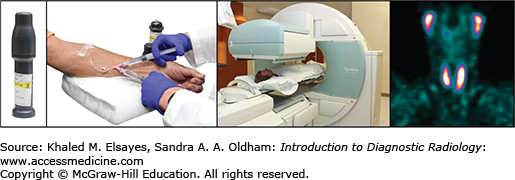
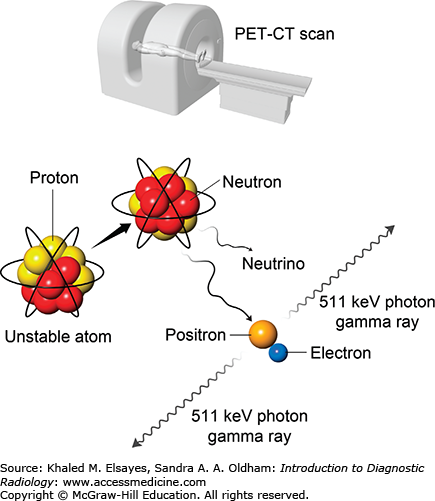
Decay of positron emitters such as fluorine-18 leads to a matter-antimatter reaction, which produces a pair of 511 keV gamma rays that travel 180° apart (Fig. 2.5). A positron emission tomography (PET) machine uses a ring of crystals to take advantage of the two rays and requires near-simultaneous detection of the rays. The result is better image spatial resolution (4 mm) than gamma cameras can achieve (as low as 7 mm for SPECT). PET studies are three-dimensional data sets typically displayed in the axial, sagittal, and coronal planes. Today, PET machines have an integrated CT scanner that provides anatomic images and quick attenuation correction (Fig. 2.6). The anatomic data provided by the CT complements the functional data leading to a higher specificity and some improvement in sensitivity in detection of many cancers. PET-MRI machines have recently come into the commercial market, and their clinical role is still being defined. Table 2.2 lists commonly used positron-emitting radionuclides in PET imaging and their physical characteristics.
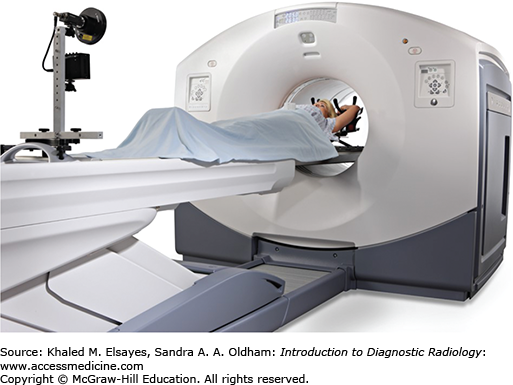
Fig. 2.6
State-of-the art PET-CT scanner manufactured by GE Healthcare, Milwaukee, WI. The PET scanner is assembled in tandem with a CT scanner and covered by a single gantry housing. The typical imaging protocol for combined PET-CT imaging includes obtaining first a CT topogram, then a spiral CT scan, followed by a PET scan.
Positron-Emitting Radionuclides and Their Physical Characteristics
| Radionuclide | Physical Half-Life (min) | Positron Energy (MeV) | Range in Soft tissue (mm) | Production Method |
|---|---|---|---|---|
| 11Carbon | 20.4 | 0.96 | 4.1 | Cyclotron |
| 13Nitrogen | 9.96 | 1.19 | 5.4 | Cyclotron |
| 15Oxygen | 2.03 | 1.73 | 7.3 | Cyclotron |
| 18Flourine | 109.7 | 0.64 | 2.4 | Cyclotron |
| 68Gallium | 68 | 1.9 | 8.1 | Generator (68Germanium) |
| 82Rubidium | 1.3 | 3.15 | 15 | Generator (82Strontium) |
| 124Iodine | 4.18 d | 2.1 | 10 | Cyclotron |
As nuclear medicine studies use ionizing radiation, the amount of radiopharmaceutical prescribed for a study should be the lowest dose able to create diagnostic images for the expected patient population of a department, termed the “as low as reasonably achievable” principle (ALARA). However, the radiation dose to the patient for many nuclear medicine studies is at the higher end of diagnostic imaging (Table 2.3). When ordering any study, the risks and benefits should be weighed. In the United States, the dose of a radiopharmaceutical is prescribed in millicuries (mCi) or microcuries (μCi). The curie is based on the number of disintegrations of 1 g of radium and is set at 3.7 × 1010 disintegrations per second. Internationally, the becquerel (Bq), defined as 1 disintegration per second, is used, and diagnostic radiopharmaceuticals doses are typically in the megabecquerel (MBq) range. One millicurie equals 37 million Bq or 37 MBq. Prescribing of radiopharmaceuticals is restricted to physicians who have undergone specialized training and fulfilled the requirements for an authorized user (AU) set out by the Nuclear Regulatory Commission (NRC) and the states. As you read about many types of nuclear medicine studies on the following pages, note the significant role nuclear medicine studies play in patient care when used for an appropriate indication.
Typical Effective Radiation Dose From Common Radiological and Nuclear Medicine Studies
| Exam | Effective Dose mSv (mrem) |
|---|---|
| Chest | 0.1 (10) |
| Cervical spine | 0.2 (20) |
| Thoracic spine | 1.0 (100) |
| Lumbar spine | 1.5 (150) |
| Pelvis | 0.7 (70) |
| Mammogram (2 views) | 0.36 (36) |
| Skull | 0.1 (10) |
| Hand or foot | 0.005 (0.5) |
| Barium enema | 7.0 (700) |
| Abdomen (KUB) | 0.7 (70) |
| CT head | 2.0 (200) |
| CT chest | 7.0 (700) |
| CT abdomen/pelvis | 10.0 (1,000) |
| Brain (perfusion) 99mTc HMPAO | 6.9 (690) |
| Bone 99mTc MDP | 6.3 (630) |
| Kidney 99mTc DTPA | 1.8 (180) |
| Kidney 99mTc MAG3 | 2.2 (220) |
| Tumor/Infection 67Ga | 2.5 (250) |
| Heart (stress-rest) 99mTc sestamibi | 9.4 (940) |
| Heart (stress-rest) 201Tl chloride | 41.0 (4,100) |
| Heart (stress-rest) 99mTc tetrofosmin | 11.0 (1,100) |
| Various PET Studies 18FDG | 14.0 (1,400) |
ENDOCRINE
As the thyroid actively transports iodine in to make the hormones T3 and T4, several isotopes of iodine (radioiodine) have been employed to diagnose and treat benign and malignant thyroid diseases. When nuclear reactor by-product iodine-131 (I-131) became available after World War II, the radioisotope was quickly brought into clinical use and helped spur the development of nuclear medicine.
Hyperthyroidism is a common disease in the United States with an incidence up to 1.3%, though the majority of cases are subclinical. Etiologies include Graves’ disease, toxic multinodular goiter, and toxic nodule. Graves’ disease has an autoimmune origin with a peak occurrence during the third to fifth decades of life and a female predominance. Toxic multinodular goiter predominantly occurs in elderly people, and the thyrotoxic state can notably worsen comorbidities. Toxic nodules are typically solitary, account for 2% to 5% of hyperthyroid patients, and arise from a mutation in the thyroid stimulating hormone (TSH) receptor signaling pathway resulting in cells that are constantly turned on and functioning independently of the normal pituitary-thyroid control mechanism. Toxic nodules need to reach approximately 3 cm in diameter before they become clinically apparent.
As iodine is an integral component of thyroid hormones, radioiodines are ideal radiopharmaceuticals as they are actively transported, trapped, and organified in the same manner as nonradioactive iodine, providing clinically relevant thyroid function data. While I-131 was initially used for imaging, the damaging beta particle emission in addition to the high-energy gamma limits the amount that can be safely used resulting in suboptimal images. Today, iodine-123 (I-123) is used for hyperthyroid imaging as there are no particulate emissions and the primary gamma emission of 159 keV is in an appropriate range for gamma cameras. Technetium-99m (Tc-99m) pertechnetate can also be used to image the thyroid as pertechnetate is trapped in the thyroid, but organification does not occur, leading to rapid washout. Due to a lower radiation dose to the thyroid, Tc-99m pertechnetate is preferentially used in children.
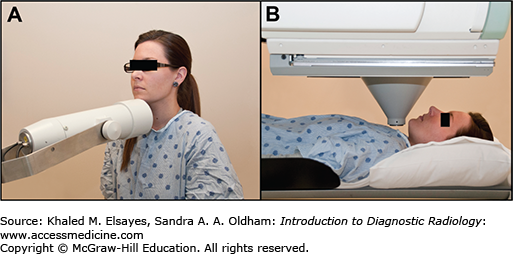
Hyperthyroid imaging consists of two parts—thyroid uptake and thyroid scintigraphy. The goal of thyroid uptake is to determine the percent of the radiopharmaceutical administered that was trapped in the thyroid. This uptake can be compared to normalized values for the local populations (iodine 10%-30% at 24 hours, Tc-99 pertechnetate 0.3%-4.5%). Also, the uptake can be used to calculate the dose for I-131 therapy of hyperthyroid patients, better tailoring the dose to their condition. I-123 uptake uses a separate device called an uptake probe consisting of a single-hole collimator coupled to a Na-I crystal to take readings of the neck as well as the thigh to allow background counts to be factored in (Fig. 2.7). Tc-99m pertechnetate uptake employs a camera-based technique that is less accurate.
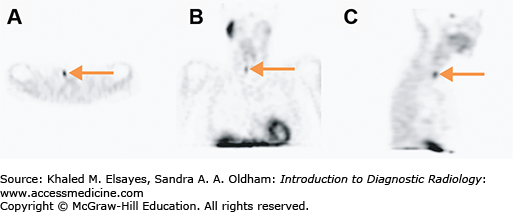
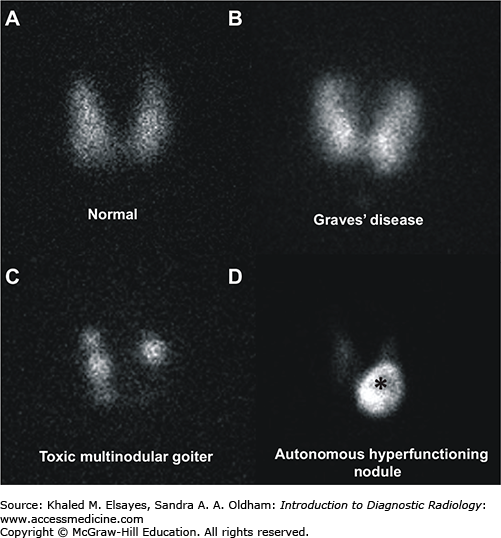
Thyroid scintigraphy is performed on a gamma camera with a pinhole collimator (large cone-shaped collimator with a single hole used to magnify structures) for I-123 and a parallel hole collimator for T-99m pertechnetate. Unlike the anatomic detail that thyroid ultrasound provides, thyroid scintigraphy details the function of the entire thyroid. In a normal thyroid, the tracer activity will be fairly homogeneous throughout the gland. In Graves’ disease, the tracer activity is also homogeneous, though more intense reflecting the greater uptake, but the contour of the thyroid becomes convex reflecting the goitrous anatomy. Multinodular goiter, both toxic and nontoxic, has rounded areas with lower uptake than a normal thyroid (photopenic areas) that corresponded to hypofunctioning thyroid nodules, rounded areas with normal uptake (or supranormal in the case of toxic multinodular), and areas of homogeneous uptake that correspond to thyroid tissue without nodules. In the case of a toxic nodule, the nodule has significant uptake while the remainder of the thyroid uptake is low to absent reflecting the suppression of the normal thyroid tissue in response to low TSH levels (Fig. 2.8).
Fig. 2.8
Common imaging patterns seen in thyroid scintigraphy. (A) Anterior pinhole image of the thyroid gland in a patient with normal thyroid profile demonstrating physiologic radiotracer activity. The 24-hour radioiodine uptake was calculated to be approximately 15% (normal range is 10%-30%). (B) Anterior pinhole image in a patient with Graves’ disease demonstrating diffuse increased radiotracer activity in the thyroid gland and elevated 24-hour radioiodine uptake of 77%. (C) Anterior pinhole image in a patient with thyrotoxicosis and an enlarged thyroid gland demonstrating areas of decreased radiotracer uptake interspersed between areas of normal and increased radiotracer activity, consistent with a toxic multinodular goiter. The 24-hour radioiodine uptake was calculated to be approximately 25%. (D) Anterior pinhole image shows increased radiotracer activity in the inferior portion of the left thyroid lobe, which corresponded to a palpable nodule on physical examination, compatible with an autonomous hyperfunctioning thyroid nodule (asterisk). The remaining portions of the thyroid gland are suppressed and have relatively decreased radiotracer uptake.
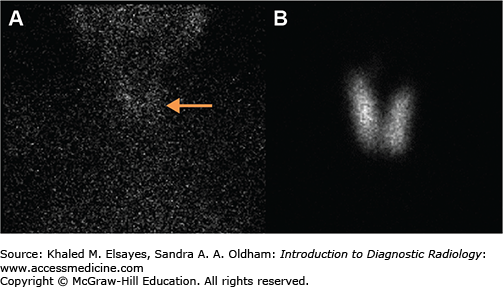
In patients with thyroiditis, thyroid hormone levels may be elevated and the TSH suppressed, however the tracer activity in the gland will be minimal to absent. Thyroid uptake will be very low, reflecting the reduced function of the inflamed thyroid gland (Fig. 2.9). A large dose of exogenous iodine demonstrates thyroid uptake and scan findings indistinguishable from thyroiditis. Therefore, a thorough history detailing diet, medication use, and recent imaging studies should be obtained from the patient. If intravenous CT contrast has recently been administered, an iodine washout period of at least 6 weeks should be observed before thyroid scintigraphy. In rare cases, hyperthyroid patients may have suppressed cervical thyroid tissue due to hyper-functioning thyroid tissue in an ovarian teratoma (struma ovarii). Imaging over the pelvis including SPECT-CT may aid in the diagnosis.
Fig. 2.9
A 41-year-old female who presented with thyrotoxicosis 2 weeks after a viral illness. (A) Anterior planar image of the neck and chest demonstrates diffuse decreased radiotracer activity in the thyroid gland, compatible with subacute thyroiditis (arrow). The 24-hour radioiodine uptake in the neck was approximately 0.3% (normal range is10%-30%). (B) Anterior pinhole image of the thyroid gland obtained 2 months after image A demonstrates normal radiotracer activity in the thyroid gland, consistent with interval improvement.
Thyroid nodules are found with a greater frequency today as they are incidentally picked up on conventional imaging studies. Whether detected by physical exam or incidentally, thyroid nodules larger than 1 cm should be evaluated by ultrasound and/or biopsy unless the TSH is suppressed. In the case of TSH suppression, thyroid uptake and scan is the most cost-effective strategy to determine whether the thyroid nodule is the etiology for the thyrotoxicosis. A photopenic nodule on thyroid scintigraphy has a 5% to 10% chance of being malignant and should be referred for ultrasound and fine needle aspiration (FNA) biopsy. Thyroid scintigraphy can also play a role after biopsy of a concerning nodule returns indeterminate results; the next step would be hemithyroidectomy. If the nodule has activity similar to or greater than the normal thyroid, the nodule has such a low likelihood of being thyroid cancer that it can safely be monitored with ultrasound, sparing the patient from undergoing unnecessary surgery.
For Graves’ disease, antithyroid medications such as methimazole and propylthiouracil (PTU) are initially given, often in conjunction with beta-blockers for symptomatic relief. However, the antithyroid medications have significant side effects that limit their long-term use. Thyroidectomy as treatment for Graves’ disease is uncommon in the United States but more widely used in iodine-deficient areas where concomitant nodules are frequently found.
Since the 1950s, I-131 has been used to effectively treat Graves’ disease, frequently with a single treatment. As the thyroid avidly accumulates most of the I-131 administered, the thyroid receives a very high dose of radiation while the remaining dose delivers a much lower dose to the rest of the body, and the unorganified I-131 washes quickly out of the body. The beta particle of I-131 has a short range, sparing surrounding tissue from high radiation doses. In the past, attempts were made to make the patient euthyroid, but the result was fluctuation between hypothyroid, euthyroid, and hyperthyroid states and multiple I-131 treatments. As the natural history of Graves’ disease ends in hypothyroidism as the gland burns out, currently I-131 doses are targeted for the rapid onset of hypothyroidism by 6 months after therapy. To plan patient-specific doses, the percent uptake from the thyroid uptake and scan and the estimated gland size from palpation are needed. After therapy, patients follow radiation safety precautions discussed in the section Thyroid Cancer for several days to reduce the dose to members of the public. The endocrinologist will monitor the TSH for hypothyroidism, typically 3 to 6 months after therapy, and then place the patient on lifelong thyroid hormone replacement therapy.
Toxic multinodular goiter and toxic adenomas are more resistant to I-131 therapy, and empiric doses from 25 to 30 mCi are frequently used. The side effects of I-131 therapy are minimal. Nausea may occur initially prior to the digestion of the pill but resolves within several hours. Pain and swelling of the thyroid are uncommon. While there has been a concern about second cancers induced by the radiation, large studies have only demonstrated a small rise in leukemia in patients treated for Graves’ disease.
The incidence of well-differentiated thyroid cancer in the United States has roughly doubled since 1990, due in part to the increased utilization of conventional imaging leading to detection of asymptomatic nodules. Most thyroid cancers occur in the young with only one-third of cases occurring after age 55. Well-differentiated thyroid carcinomas such as papillary and follicular carcinomas retain the ability to concentrate iodine, though at a lower rate than normal thyroid tissue. Anaplastic thyroid cancer is too dedifferentiated to concentrate iodine, and medullary thyroid carcinoma arises from parafollicular cells that do not concentrate iodine.
Surgical removal of the thyroid and local lymph nodes is the initial step in treatment. If the thyroid cancer is 1 cm or less in size with no lymph node metastases, further therapy with I-131 has not demonstrated a survival advantage or reduction in relapse rate. Larger thyroid cancers and those with metastases can benefit from I-131 therapy.
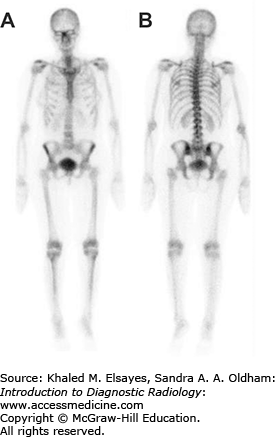
Approximately 6 weeks after thyroidectomy, while the patient is on a low iodine diet, a whole-body study with I-131 or I-123 surveys the body for metastases and allows uptake measurements of the neck to be obtained to ensure that significant amounts of thyroid tissue that could interfere with I-131 therapy do not remain (Fig. 2.10). The dose of I-131 prescribed by an authorized user is based on the pathologic features of the tumor including size and extracapsular spread, lymph node metastases, and extranodal metastases (lung, bone). For less complex cases, an empiric set of doses ranging from 75 to 200 mCi is frequently used. For more complex cases, the whole-body scan can be used to determine a higher dose of I-131 that does not give excessive radiation to the bone marrow.
Fig. 2.10
A 69-year-old female with differentiated thyroid carcinoma status post total thyroidectomy. (A) Anterior and (B) posterior whole-body planar images and (C) anterior and (D) posterior spot images of the neck and thorax, obtained 72 hours after the oral administration of 5 mCi of sodium I-131, demonstrate a focus of increased radiotracer activity in the left thyroid bed (arrows), compatible with residual thyroid tissue. Physiologic activity is seen in the nasal region, salivary glands, stomach, bowel, and urinary bladder. The radioiodine uptake in the thyroid bed was 0.8% and the patient was subsequently administered 100 mCi of I-131 orally for thyroid remnant ablation.
Patients undergoing I-131 therapy need to follow radiation safety precautions to reduce the dose to themselves and others for 1 to 2 weeks. Most patients can safely be released after therapy, but some patients need to be admitted for several days to a lead-lined room in the hospital as their home situation does not allow for release. As I-131 is excreted in the fluids of the body, particularly the urine, patients are instructed to drink enough fluids to void every couple of hours, thereby reducing the dose to the bladder and clearing the circulating iodine from the body. For the duration of the precautions, patients should use a bathroom and shower separate from the rest of the household. Patients should maintain their distance from other people, especially children, who are more radiosensitive. As the dose of radiation falls off with the square of the distance, relatively small changes in distance result in large reductions in radiation doses. Women who are pregnant cannot be treated with I-131 due to the radiation exposure to the fetus and the possible damage to the fetal thyroid. It is not recommended that the patient become pregnant for at least 6 months after treatment.
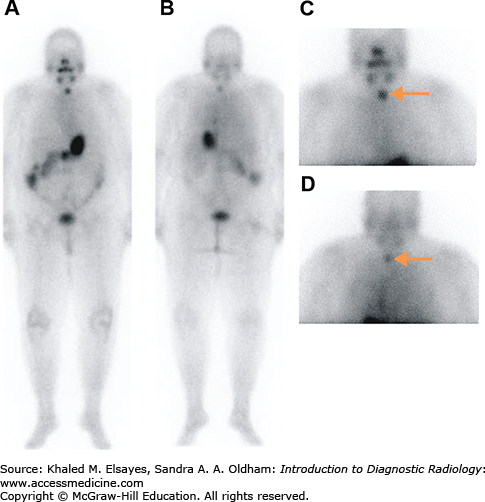
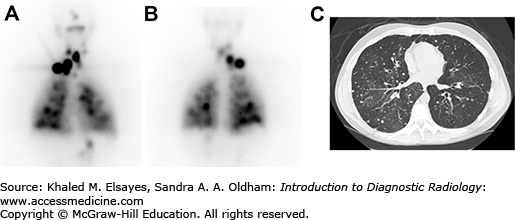
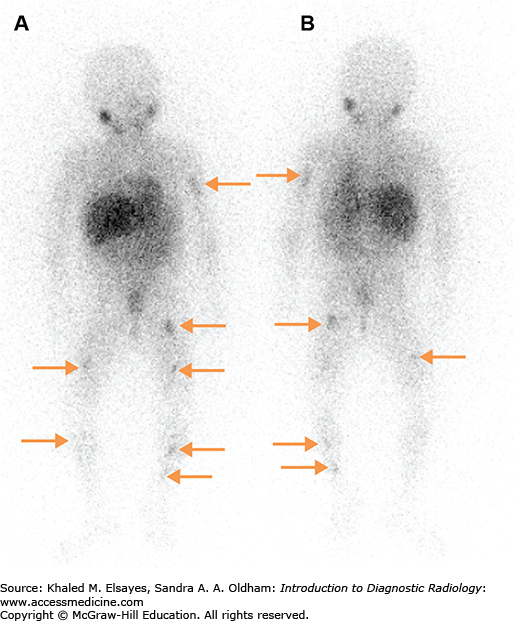
Seven days after therapy, the patient returns for whole-body imaging using the I-131 remaining in the body after therapy to survey for metastases (Fig. 2.11). After the I-131 dose has destroyed the remaining normal thyroid tissue and hopefully treated any metastatic disease, the blood level of thyroglobulin, a protein made by thyroid tissue, is followed as it is a sensitive marker for thyroid cancer recurrence. If the thyroglobulin rises, ultrasound of the neck can be used to detect cervical lymph node metastases. Whole body scans with radioiodine can also be used to assess for metastases. Patients can receive multiple doses of I-131 for treatment of thyroid cancer, though if above a lifetime dose of 600 mCi, the incidence of leukemia increases. The thyroid cancer can dedifferentiate over time, losing the ability to concentrate iodine and becoming refractory to I-131 therapy. When the cancer becomes iodine negative on the whole body scan, F-18 fluorodeoxyglucose (FDG) PET-CT is useful in assessing the extent of disease and the presence of FDG-avid thyroid cancer is an independent risk factor for poor prognosis.
Fig. 2.11
(A) Anterior and (B) posterior planar images obtained 7 days after the oral administration of 200 mCi of sodium I-131 to a patient with metastatic papillary thyroid carcinoma. There are multiple foci of increased radiotracer uptake in the neck, mediastinum, and lungs bilaterally, compatible with radioiodine avid metastases. (C) Axial CT image demonstrating multiple pulmonary nodules bilaterally, consistent with lung metastases.
Hyperparathyroidism is generally discovered on routine lab work in asymptomatic patients as opposed to the late symptomatic presentations in the past. Primary hyperparathyroidism accounts for 80% of the cases, and 85% of patients with primary hyperparathyroidism have adenomas as the etiology. Parathyroid scintigraphy is directed at detecting adenomas as normal and hyperplastic glands are too small to reliably imaged. Secondary and tertiary hyperparathyroidism arise in the setting of severe renal dysfunction that leads to parathyroid hyperplasia and eventually autonomous function. Surgery is the treatment of choice.
Several radiopharmaceuticals have been used over the years to image parathyroid adenomas. Currently, a protocol using Tc-99m sestamibi imaged at 10 minutes and 2 hours is employed. On early images, both the thyroid and parathyroid tissue demonstrate uptake, but the thyroid activity washes out by 2 hours. Planar images must include the area from the parotid glands to the top of the heart as ectopic parathyroid adenomas found within this range are the cause of the hyperparathyroidism in less than 5% of cases. The sensitivity for adenomas 300 mg or more in size (roughly 10 times the weight of a normal gland) is greater than 85%. SPECT-CT is frequently performed to increase sensitivity and to aid in surgical planning (Fig. 2.12).
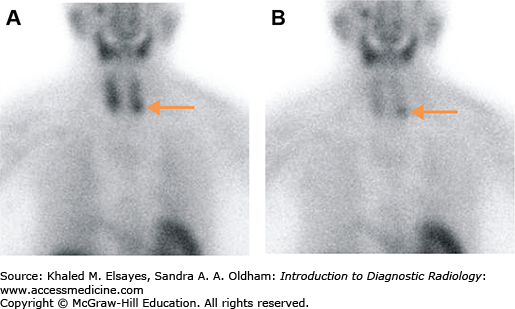
Fig. 2.12
A 79-year-old female with hypercalcemia and increased serum parathyroid hormone (PTH). (A) Immediate anterior planar image demonstrates a focus of increased radiotracer activity near the inferior pole of the left thyroid lobe, which persists on the delayed planar image (B). This finding is suggestive of a parathyroid adenoma (arrow). Please note the relative washout of radiotracer from the normal thyroid gland on the delayed image.
Neuroendocrine tumors arise from tissues originating embryologically from neural crest cells and include pheochromocytomas, paragangliomas, gastric endocrine tumors (eg, carcinoid), and medullary thyroid carcinoma. Small cell lung cancer also falls in this category but is primarily imaged by FDG PET-CT. Both benign and malignant neuroendocrine tumors can be imaged with nuclear medicine studies.
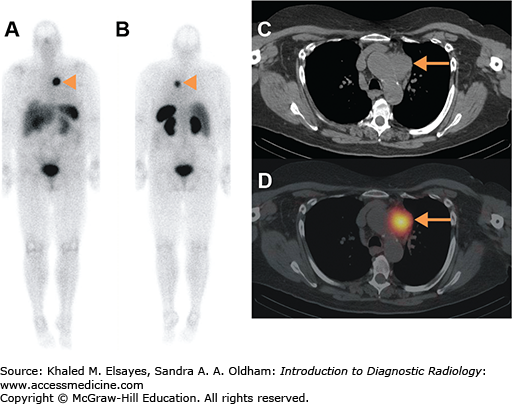
Many neuroendocrine tumors overexpress somatostatin receptors. Somatostatin is a hormone with inhibitory effects on multiple pathways. The radiopharmaceutical indium-111 (In-111) pentetreotide binds to somatostatin receptors 2, 3, and 5, allowing a wide variety of neuroendocrine tumors to be imaged with a high sensitivity. However, some tumors such as insulinomas and medullary thyroid carcinoma are not imaged well with pentreotide. As there is significant physiologic renal, hepatic, and splenic radiotracer uptake, SPECT-CT is routinely performed after whole-body imaging to better evaluate the upper abdomen or lesions of interest seen in planar images (Fig. 2.13). For carcinoid tumors, if the pentreotide study is positive, this is good evidence that the patient will respond to Sandostatin therapy.
Fig. 2.13
A 54-year-old female with metastatic well-differentiated neuroendocrine carcinoma (carcinoid tumor), referred for restaging. (A) Anterior and (B) posterior whole-body planar images obtained 4 hours after the intravenous administration of 6 mCi of In-111 pentetreotide demonstrating an area of increased radiotracer activity in the mediastinum, compatible with biopsy-proven nodal metastasis (arrowheads). (C) CT and (D) fused SPECT-CT images of the chest demonstrate increased radiotracer activity localizing to a nodal mass in the prevascular region (arrows).
Neuroendocrine tumors arising from the adrenergic tissues such as pheochromocytomas and paragangliomas are preferentially imaged with meta-iodobenzylguanidine (MIBG), which concentrates in presynaptic adrenergic nerves. MIBG is also used in the evaluation of neuroblastoma, a malignant neoplasm occurring primarily in children less than 4 years old (Fig. 2.14). The sensitivity for adrenergic tumors is greater than 90% with a similarly high specificity. Many drugs interfere with the uptake of MIBG, so a thorough history must be obtained from the patient prior to imaging.
High-dose I-131 MIBG has been used as a palliative treatment for metastatic neuroendocrine cancers. Due to the high doses used, all patients are admitted to an appropriately shielded hospital room until the level of radiation being emitted from the patient is safe for the public. In addition, MIBG has been used to image the adrenergic innervation of the heart both in congestive heart failure and after heart transplant.
CARDIOVASCULAR SCINTIGRAPHY
Roughly half of the 16 million nuclear medicine procedures performed each year in the United States are cardiac imaging studies. Nuclear medicine studies have the advantage of being noninvasive measurements of several cardiac functions that still provide valuable information in addition to echocardiography, cardiac MRI, and even cardiac catheterization.
Fundamentally, myocardial perfusion imaging (MPI) detects differences in blood flow to the left ventricle and myocardial extraction of tracers when radiopharmaceuticals are injected during rest and stress conditions caused by a significant coronary artery stenosis. Both SPECT and PET studies are performed. While PET has a higher sensitivity and specificity at 90% and 88% versus 85% and 85% for Tc-99m SPECT, the application of PET is generally limited by cost to patients who are obese and those with an inconclusive MPI SPECT study [1]. Exercise treadmill tests alone have a sensitivity of 75% with many false positives, particularly in women (specificity around 60%) and patients with certain baseline electrocardiography (ECG) abnormalities.
MPI SPECT can be performed with two radiopharmaceuticals, thallium-201 chloride (Tl-201) and Tc-99m agents, in a wide variety of protocols beyond the scope of this text. Tl-201 is a physiologic potassium analogue that is actively transported into myocardial cells with a very high first-pass extraction. Tl-201 is not bound in the cell and redistributes over time into all viable myocardium independent of the blood flow conditions under which it was injected. Due to a long physical half-life of 3 days coupled with a 10-day half-life in the body, the amount of Tl-201 used for imaging is low (up to 4 mCi). Also, the primary imaging photons are x-rays in the range of 69 to 83 keV, which is lower than the optimal range for gamma camera leading to the worst image quality of MPI radiotracers.
The Tc-99m agents, sestamibi and tetrofosmin, have a lower first-pass extraction than Tl-201 but do not redistribute as they are trapped within mitochondria. Tc-99m has a 6-hour half-life allowing for higher dose usage than Tl-201 and is in the optimal range for gamma cameras leading to better image quality than Tl-201 and making ECG-gated images more feasible.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree