INTRODUCTION TO INTERVENTIONAL RADIOLOGY
Interventionalist radiologists are trained in performing diagnostic and therapeutic interventions in patients. This requires expertise in imaging, technical skills, pharmacology, sedation/anesthesia, and management of patients preprocedure and postprocedure. A well-rounded interventionalist understands disease processes and how they adversely alter normal physiology. They use this knowledge to work up the patient appropriately, use different imaging modalities to tailor a custom treatment for each patient, skillfully and safely perform the procedure, and follow up the patient to ensure optimal outcome.
This chapter is a basic outline of Interventional Radiology (IR) meant to introduce medical practitioners in other fields to the practice of IR. It contains the most common techniques, tools, and procedures involved in IR today. IR practice is diverse, innovative, and flexible. It is based on the practitioner preferences, training, experience, and ongoing literature review. The procedures covered in this chapter are performed commonly and may vary depending on the institution and interventionalist performing them.
The workup starts with a thorough history and physical, with particular attention to pertinent medical and surgical history, history of allergies, current medications, and a focused physical exam. It is necessary to identify risk factors for contrast allergy, contrast-induced nephropathy, and bleeding.
Previous reaction to a contrast agent and a history of allergies or asthma place the patient at a higher risk for an allergic reaction to contrast during or after the procedure. Contrast reactions are covered in chapter 1 of this book. Pretreatment with a histamine blocker like diphenhydramine and a steroid such as prednisone can help reduce the risk of contrast reaction during the procedure. Low-osmolar contrast agents and carbon dioxide angiography can be used in patients with a history of moderate to severe contrast reaction. It is important to discuss alternative treatments or risk versus benefit of using contrast during an intervention with the patient prior to starting the procedure.
CIN is defined as an increase in baseline creatinine by either 0.5 mg/dL or 25% after 1 to 3 days following administration of contrast. The renal function generally returns to baseline after a week. Pharmacologic agents such as N-acetylcysteine (Mucomyst), use of a limited volume of low osmolar contrast agents, carbon dioxide as an alternative contrast agent, and preprocedural and postprocedural hydration with saline can lower the risk of CIN.
Routine laboratory tests include prothrombin time (PT), activated partial thromboplastin time (PTT), platelets, and international normalized ratio (INR). Table 8.1 illustrates safety threshold and steps to rectify abnormalities of these parameters.
Important Preprocedural Hematologic Parameters
| Parameter | Safety Threshold | Measures to Correct Abnormalities |
|---|---|---|
| PT/INR | < 3 s/1.6-1.8 | Withhold coumadin, administer fresh frozen plasma or vitamin K |
| PTT | < 6s | Withhold heparin 2-6 h prior to procedure |
| Platelet | > 50,000 | Platelet transfusion |
| Bleeding time | < 8 s | Administer cryoprecipitate, platelet transfusion |
Antiplatelet agents such as aspirin or clopidogrel are held for 5 to 7 days before the procedure. Hemoglobin and hematocrit level is also documented in patients with an increased chance of bleeding due to a coagulopathy or angiography and biopsy of vascular organs or lesions.
Prior to any procedure, an informed consent must be obtained. To provide this, the patient must understand the procedure planned, reasons for undergoing the procedure, risks and benefits, and alternative therapies including consequences of refusing the procedure. The consent also includes the risk of CIN and allergic reaction to contrast agents. Sedation and anesthesia are also addressed as part of the consent.
If sedation or anesthesia is planned, the patient is to be NPO (nothing by mouth) for 8 hours prior to the procedure. Medications such as antihypertensives and insulin should not be discontinued prior to the procedure. Antibiotics are given within 2 hours of procedures involving the biliary system, genitourinary system, drainage of abscesses, transjugular intrahepatic portosystemic shunt or endograft placement, and tissue ablative therapies.
During the procedure, the interventionalist takes steps to reduce radiation exposure to the patients and himself or herself by using techniques such as collimation, reduced fluoroscopy time, pulsed fluoroscopy, reduced patient to image intensifier distance and increased distance from the radiation source, use of lead shields, and wearing protective gear. Excessive radiation can cause tissue damage in the short term and increase the chance of malignancies in the distant future.
Steps are taken to prevent blood-borne pathogen (hepatitis B or C, HIV) exposure to the interventionalist and patient. Appropriate precautions are taken before, during, and after the procedure to minimize the risk of exposure. The patient’s vital signs are monitored by a dedicated nurse throughout the procedure.
Anxiolytics and pain medications are used during the procedure to lessen patient discomfort. A benzodiazepine such as midazolam (anxiolytic and antegrade amnesia) and an opioid narcotic such as fentanyl are most commonly used to establish moderate sedation (patient is responsive to verbal commands and maintains airway protection reflexes). Procedures that are particularly painful or that involve the pediatric population are performed with general anesthesia established by an anesthesiologist.
One should anticipate, understand, and be ready to manage/treat intraprocedural events such as hypotension, respiratory depression with hypoxia, vasovagal reactions (hypotension with bradycardia, nausea, and diaphoresis), hypertension, hemorrhage, and contrast reactions. Interventionalists are also expected to be proficient at advanced cardiovascular life support (ACLS) in the event of a cardiopulmonary arrest.
Postprocedural care is dictated by the type of procedure performed. Needle punctures for biopsies, aspiration of fluid, and venous access can generally be managed by applying manual digital pressure directly on the puncture site for a few minutes to achieve hemostasis, followed by application of a sterile dressing.
Arterial punctures with placement of sheaths require application of digital pressure above, at, and below the arteriotomy site for at least 15 minutes post sheath removal. The site needs to be carefully monitored for enlarging hematoma. The patient should be on bed rest with the ipsilateral extremity immobile for 6 hours. Use of arterial closure devices has the advantage of hemostasis almost immediately following deployment and much shorter bed rest time.
Solid organ biopsies (liver, spleen, and kidney) have a higher chance of bleeding and the patient is monitored for 3 to 4 hours post procedure for signs of bleeding. Lung biopsies are followed by immediate and 3-hour chest x-rays to monitor for a pneumothorax, which may need to be evacuated by chest tube placement in symptomatic patients.
Specific orders regarding activity, diet, pain control, hydration, and monitoring vital signs are also written. Discharge or transfer criteria include tolerance of oral intake, adequate pain control, appropriate mentation, and stable vital signs.
Inpatient and outpatient follow-up should be arranged based on the procedure performed. It is an integral part of being a well-rounded interventionalist. Some treatments are done in a staged fashion, requiring long-term follow-up.
COMMON TECHNIQUES AND INSTRUMENTS
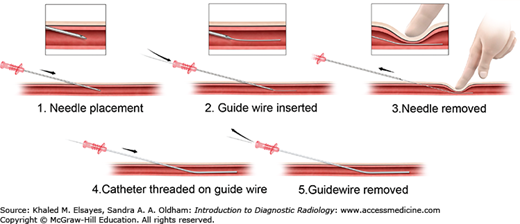
The Seldinger technique (Fig. 8.1) is used on a daily basis for percutaneous arterial catheterization. It involves the use of a needle, guidewire, and catheter. Vascular access is most commonly attained via the common femoral artery. Using the femoral head and inguinal ligament as a landmark under fluoroscopy, an appropriate skin entry site in chosen. Local anesthetic is given using 1% or 2% lidocaine at the beginning of the procedure in an intradermal and subcutaneous fashion. Aspiration prior to injection is used to avoid intravascular injection of lidocaine.
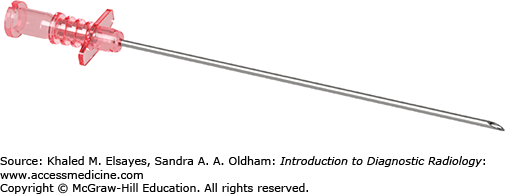
A superficial skin nick is made directly over the arterial pulse. Using a blunt hemostat, subcutaneous dissection is performed. While palpating the femoral artery pulse, an 18 gauge needle (Fig. 8.2) is advanced in a controlled fashion at a 45 degree angle toward the femoral head. Brisk pulsatile bright red blood is encountered upon a successful arterial puncture. A 0.035-inch Bentson wire is advanced through the needle into the common femoral artery and abdominal aorta under fluoroscopic guidance. While holding pressure at the arteriotomy site, the needle is slowly removed and the wire is cleaned with wet gauze. A sheath or catheter is then advanced over the guidewire into the common femoral artery in a smooth fashion. The guidewire and sheath inner stiffener is removed and the sheath is flushed with normal saline. The sheath can then be used to exchange for different types, sizes, and length of catheters. Catheters are always advanced over a guidewire to prevent arterial injury.
Some practitioners prefer a 21 gauge needle and thinner 0.018-inch wire to access the common femoral artery. This can then be upsized to a 0.035-inch Bentson wire using a 5 French (Fr) sheath (1 Fr = 1/3 mm). Complications of this procedure include:
Retroperitoneal hemorrhage if the arterial access is too high.
Low arterial access into the superficial or deep femoral artery increasing the chance of arterial thrombosis, pseudoaneurysm, or arteriovenous fistula formation.
Arterial injury, dissection, or occlusion. The Seldinger technique is also used for central venous access.
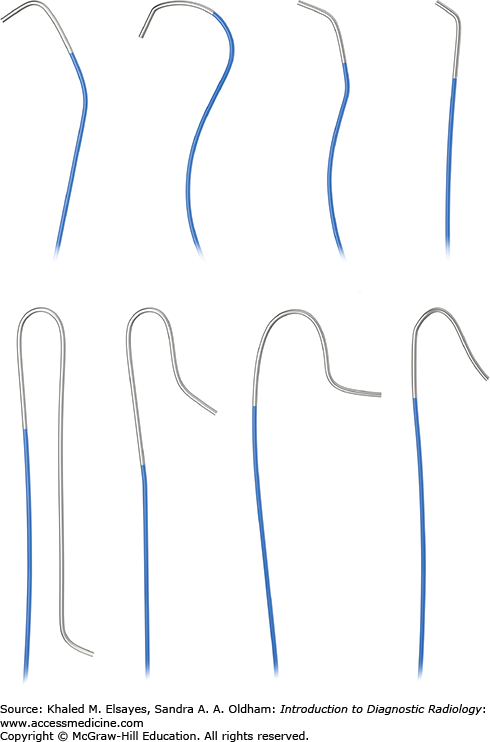
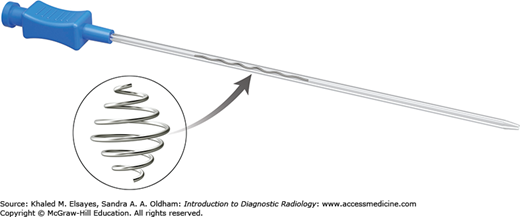
Guidewires, as the name implies, are used primarily to direct catheters into specific locations within a blood vessel, cavity, or organ. Guidewires mainly differ by length, diameter, stiffness, tip characteristics, and pliability. The exterior may be hydrophobic or hydrophilic. Standard guide wires come in 0.035 or 0.038-inch diameters. Microwires are available for microcatheters or small gauge needles. Choice of guidewire is based on the preference of the interventionalist and procedure being performed.
There are angiographic and drainage catheters (Figs. 8.3–8.5). Angiographic catheters differ by pliability, external coating, and tip contour.
Reverse curve catheters are used to access vessels and need to be formed into the correct shape inside the vessel. They can also be shaped by the interventionalist.
Pigtail catheters are used for angiography or venography of large vessels and for drainage of air or fluid. They have multiple side holes along the distal shaft and pigtail loop. Drainage catheters have a string that is pulled to secure the shape of the pigtail loop to prevent dislodgement.
Guiding catheters have a specific shape that can help cannulate tortuous or angled vessels.
Microcatheters are used in select small caliber vessels.
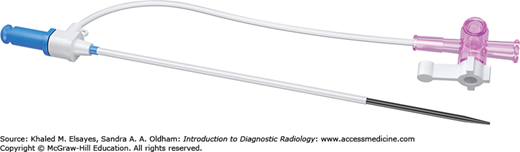
Sheaths (Fig. 8.6) are placed when lengthy vascular interventions and multiple catheter/guidewire exchanges are anticipated. They provide a safe passage of guidewires and catheters into the artery. Sheaths can also be placed to control persistent oozing or hematoma at the arterial access site. Sheaths are composed of an inner stiffener and outer thin wall. The inner stiffener is present to prevent buckling during insertion and is removed once access has been established. They also have a valve at the proximal end to prevent free backflow. A side arm is available for injection of contrast. Some have a peel-away outer component that can be easily removed once a more stable access to a vein, organ, or cavity has been established. Vascular sheaths can also be used for nonvascular procedures.
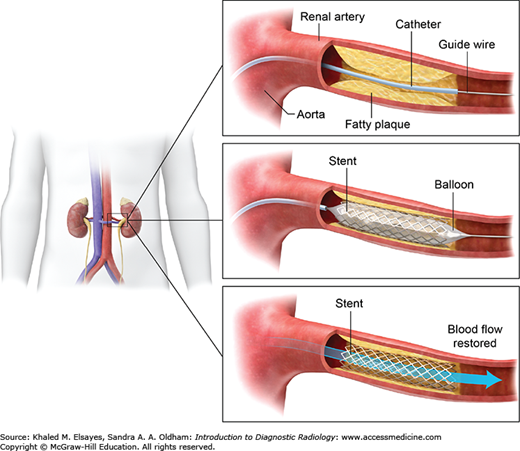
Inflatable balloons are used to open stenosis within vessels, or other parts of the body such as the urinary tract, and biliary systems. The procedure is called percutaneous transluminal balloon angioplasty (PTA). Using an inflation device, the balloon can be inflated or deflated to a measurable pressure using air, saline, or contrast. It causes a controlled stretch injury to the lumen, which then scars into place. Balloons differ by diameter, length, shape, burst pressure, surface, and pliability. Balloons are also used to expand stents placed within vessels and channels. They are inflated within the stent to achieve the desired lumen diameter and force the stent to appose the wall of the vessel or channel.
Stents are used in a variety of procedures to maintain luminal patency within arteries, veins, bile ducts, bowel, urinary tract, and artificially created channels. Stents are tubular metallic cages that differ by length, longitudinal flexibility, elasticity or plasticity, radial force, composition, radiopacity, shortening with deployment, and compatibility with magnetic resonance imaging (MRI). There are self-expanding, balloon-expandable (Fig. 8.7), drug-eluting, and covered stents. Choice of stent of varies by procedure and comfort level of the interventionalist.
Several options are available to achieve occlusion of vessels, aneurysms, biliary tract, fistulas, ureters, and vascular malformations. The choice of agent used depends on whether temporary versus permanent and proximal versus distal occlusion is desired. It also varies by the type of procedure, availability of materials, comfort level of the interventionalist, and part of the body.
Coils are used for permanent vascular occlusion (Fig. 8.8). The selection of coil depends on the diameter and length of the vessel.
Gelfoam is a spongelike agent that expands upon contact with fluid. It is used for temporary vascular occlusion of vessels or bleeding tracts. It can be made into slurry or cut into small pledgets and then injected into the desired location via a catheter.
Polyvinyl alcohol (PVA) particles cause inflammatory reaction in the vessel wall and are used to occlude small arteries or arterioles.
Embospheres and PVA microspheres are spherical agents that occlude small vessels to achieve a permanent thrombosis.
Absolute ethanol is a liquid agent that scleroses the vessels walls and surrounding tissue for permanent occlusion. Small volumes are usually sufficient, and it may cause pain after embolization.
Onyx is a liquid agent that is typically used in neurovascular interventions to treat arteriovenous malformations and endoleaks after endovascular aneurysm repair.
Avitene is fibrils that are suspended in solution and result in an inflammatory reaction that achieves permanent occlusion in vessels.
Glue is an adhesive liquid agent that achieves permanent occlusion of arteriovenous malformation, fistulas, aneurysms, varicoceles, and gastrointestinal bleeding.
Detachable balloons can be used to occlude arteries and arteriovenous lesions.
Needles come in varying lengths, diameters (gauges), and tips. They can be used to access vessels or organs, aspirate fluid from cavities, and biopsy masses. The type of needle used depends on the procedure being performed. References will be made to one-step and two-step needles in this chapter.
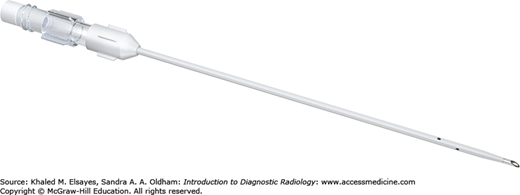
One-step needles (Fig. 8.9) consist of an inner hollow metallic needle and outer thin sheath with side holes at the distal end. They can be used to drain or access fluid within the peritoneal or thoracic cavities.
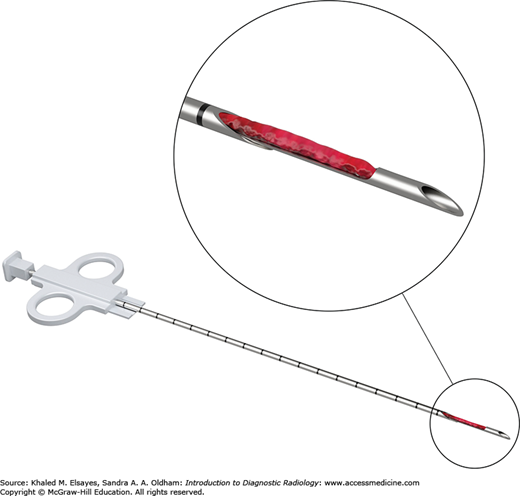
Noncutting and cutting needles are typically used for biopsies. A small sample can be aspirated into the needle attached to syringe or automatically pulled into the needle via capillary action or manual aspiration. A two-step needle (Fig. 8.10) is used for soft tissue mass biopsies and typically consists of a slotted inner stylet that catches the specimen and spring-loaded cutting outer cannula. The stylet is advanced into the biopsy target and the cutting outer cannula is manually fired. The outer cannula is then retracted to reveal the specimen within the slot.
CASE 1: ARTERIAL ANEURYSMS
An 80-year-old male presents to the ED with mild abdominal pain and pulsating midline mass on abdominal exam. The patient is hemodynamically stable.
Abdominal aortic aneurysm (AAA)
Advantages: CT angiogram is readily available and accurately demonstrates the size and shape of the AAA and its relationship to branch arteries and aortic bifurcation. It can also assess for aneurysm rupture, impending rupture, or leaking aneurysm, as well as determine the type of aneurysm. This information is essential for surgical or interventional planning. Ultrasound examination can be done at bedside and is an even quicker and cheaper modality without radiation exposure. MRI/MRA is expensive, slow, and not readily available. Noncontrast MRI has the advantage of delineating arterial anatomy better than a noncontrast CT examination in a patient with renal dysfunction.
CT findings of abdominal aortic aneurysm:
Abdominal aorta dilated to greater than 3 cm
Retroperitoneal hematoma that may involve the periaortic, pararenal, or perirenal spaces or psoas muscle with aneurysm rupture.
Treatment of a ruptured aortic aneurysm is typically done surgically in a hemodynamically unstable patient. Endovascular graft repair is ideally performed in stable patients with a nonruptured aortic aneurysm and favorable arterial anatomy, and in patients who are poor candidates for surgery due to comorbidities.
Several subtypes and complications of abdominal aortic aneurysm have been described; some of these have a prognosis that is significantly worse than others:
True aortic aneurysm: This refers to dilatation of the aorta due to weakening of the aortic walls. All three layers of the aortic wall (intima, media, and adventitia) are involved. This is the most common type of aneurysm, and atherosclerosis is the most common cause. Imaging usually shows fusiform dilatation of the aorta with atherosclerotic calcification and eccentric thrombus. Treatment depends on the size of the aneurysm and growth rate, both of which directly correlate with risk of rupture. Aneurysms larger than 5 cm in diameter in women and 5.5 to 6 cm in size in men have an annual risk of rupture of around 15%. Aneurysms greater than or equal to 8 cm in diameter have an annual rupture rate of up to 50%. A growth rate of 3 to 4 mm/y is typical for aneurysms, and a growth rate of greater than 10 mm/y warrants intervention.
Pseudoaneurysm: This is a false aortic aneurysm, characterized by disruption of the aortic wall layers with blood inside the aorta contained by only a thin layer of adventitia or surrounding soft tissue (Fig. C1.1). This can be caused by an insult to the aorta from trauma, infections, instrumentation, or inflammation. Pseudoaneurysms are at a high risk for rupture regardless of their size. They are usually saccular in shape with a defined neck. These usually require immediate treatment with endovascular stent graft placement or surgical repair.
Dissecting aneurysm: These are considered pseudoaneurysms due to a lack of confinement by all three layers of the vessel wall. The aortic dilatation is caused by splitting or dissection of an arterial wall by blood entering through an intimal tear or by an intramural hematoma; these are more frequent in the thoracic aorta where the dissection starts in the proximal aorta and descends into the abdominal aorta. Patients with an increased risk of dissection include those with a history of connective tissue disorders such as Marfan syndrome, hypertension, or advanced atherosclerotic disease with penetrating aortic ulcers. On imaging, one can see the intimal flap with true lumen and blood/ contrast in the false lumen.
Mycotic aneurysm: These are infectious in etiology and are considered pseudoaneurysms (Fig. C1.2). There is usually hematogenous spread of infection from a source such as endocarditis or a psoas or vertebral body infection. They are more common in the thoracic aorta, suprarenal aorta, or at the branch point. Imaging findings include a saccular shape, lobular contours, and aortic/periaortic inflammation or gas. The risk for rupture is very high.
Aneurysmal dilatation of the aorta can also be seen in people with inflammatory conditions such as periaortic retroperitoneal fibrosis, autoimmune disorders such as rheumatoid arthritis, and arteritis. These are usually symptomatic with patients having fever, weight loss, and hydronephrosis from ureteral involvement. An increased risk of rupture is present irrespective of size.
CASE 2: ARTERITIS
A 25-year-old Asian female presents to the ED with cold and pulseless upper extremities.
Takayasu’s arteritis
Advantages: Angiography accurately demonstrates the vessels involved and the extent of the disease in large and medium sized vessels. Immediate intervention can be performed if necessary. CT angiogram or MR angiogram is better for small vessels because it can show the effect of the disease on the visceral organs. PET/CT has a limited role and can show areas of active inflammation. Angiographic findings in arteritis include vascular stenosis, obstruction, aneurysmal dilatation, or rupture.
Several types of arteritis and their complications have been described depending on their characteristics and the vessels involved:
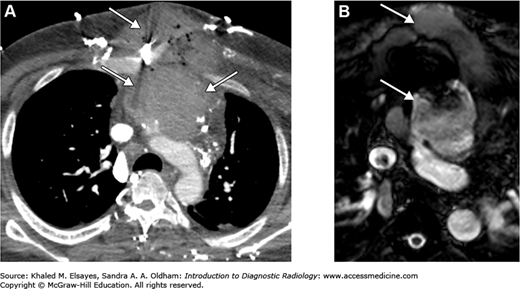
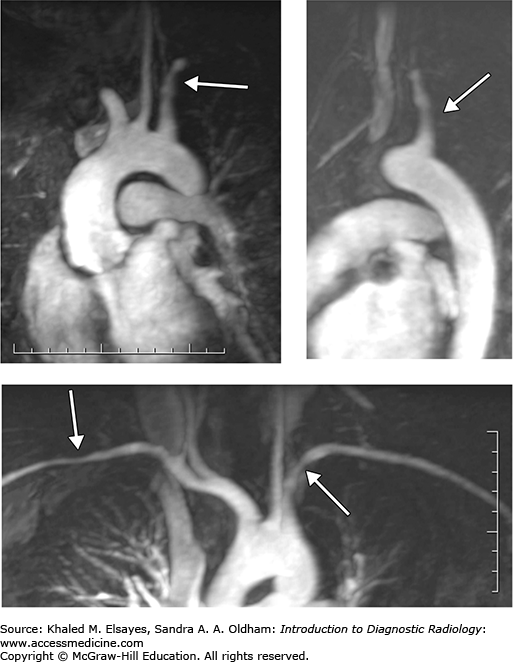
Takayasu’s arteritis: This is a large-vessel vasculitis that is a panarteritis. It typically involves the thoracic aorta and its branching vessels but can affect the pulmonary and coronary arteries as well as the abdominal aorta (Figs. C2.1 and C2.2). Aortic branches most commonly involved include left common iliac, carotid, left subclavian, renal, and superior mesenteric arteries. Dilatation of the aorta is due to weakening of the aortic walls. Inflammation of the vessel wall results in wall irregularity, diffuse vessel stenosis, occlusion, and even aneurysms. The typical patient is a young adult, particularly of Asian descent, with upper extremity claudication, fever, malaise, or abdominal pain from mesenteric ischemia.
Giant cell arteritis: This is a large vessel vasculitis also known as temporal arteritis. It is typically seen in adults older than 50 years of age who present with jaw claudication, sensitivity of the scalp, transient ischemic attacks, and visual symptoms. Angiography shows irregularity and stenosis of the superficial temporal artery. Arterial biopsy is usually performed to confirm the diagnosis.
Polyarteritis nodosa: This is a small- to medium-vessel necrotizing vasculitis typically involving the renal and mesenteric vessels. It is seen in middle-aged adults who present with fever, abdominal pain, fibromyalgia, and erythema nodosum. Imaging shows aneurysms up to 1 cm in size typically at vessel bifurcations and pseudoaneurysms of intrarenal arteries. This condition is fatal if not treated.
Kawasaki disease: Also known as mucocutaneous lymph node syndrome, this is a medium-vessel vasculitis that is largely seen in children under 5 years old. They may present with fever, conjunctival inflammation, erythematous lips, “strawberry” tongue, and cervical lymphadenopathy. Patients can also develop coronary artery aneurysms that may thrombose or rupture leading to myocardial infarction. Echocardiography and angiography can be used to detect these aneurysms.
Henoch-Schönlein purpura: This is a hypersensitivity related vasculitis affecting small vessels. It is usually seen in children and young adults who present with skin rash, lower extremity purpura, hematuria, arthritis of large joints, and bowel inflammation. CT of the abdomen may show bowel and mesenteric inflammation, enlarged mesenteric lymph nodes, intussusception, ascites, and renal pelvis suburothelial hemorrhage.
Behcet’s disease: This chronic inflammatory disease involves multiple organ systems. It is typically seen in 20- to 40- year-old patients who present with oral and genital aphthous ulcers, uveitis, and arthritis. Abdominal pain and bowel perforation can occur as well. Imaging shows occlusion and aneurysms of the pulmonary artery, aortic arch, subclavian arteries, and coronary arteries. CT of the abdomen may show masslike, polypoid thickening and enhancement of the bowel wall mucosa, bowel perforation, or fistulas.
Wegener’s granulomatosis: This is a small-vessel vasculitis. These patients typically present with renal failure, proteinuria, and hematuria, but the disease can affect the nose, lungs, and central nervous system. It is mainly seen in middle-aged adults. Imaging findings include erosions of the bony nasal septum, cavitary lung lesions, microaneurysms, renal scarring/hemorrhage, and bowel ischemia.
Lupus vasculitis: This is a necrotizing small-vessel vasculitis typically seen in young women. The superior mesenteric artery is most commonly affected, but any part of the gastrointestinal tract can be affected. Imaging shows bowel wall inflammation, pneumatosis from ischemia, engorged mesenteric vessels, ascites, mesenteric lymphadenopathy, and mesenteric thrombosis.
Buerger’s disease: Also known as thromboangiitis obliterans, it is typically seen in smokers and affects small- and medium-sized vessels in the extremities leading to vascular occlusion and limb claudication. On angiography, there are multiple symmetrical segmental stenoses and occlusions in the distal arteries of the upper and lower extremities. These arteries lack proximal atherosclerosis and have inadequate collateral vessels described as “corkscrew collaterals.”
There are other types of vasculitides due to a primary disease or related to inflammatory conditions, infection, malignancy, and drug hypersensitivity, sequelae of which can be seen on imaging. Treatment for vasculitis is based on steroids and immunoregulatory agents such as cyclophosphamide.
CASE 3: ADRENAL VEIN SAMPLING
A 45-year-old female presents with hypertension and hypokalemia. Hyperaldosteronism is suspected.
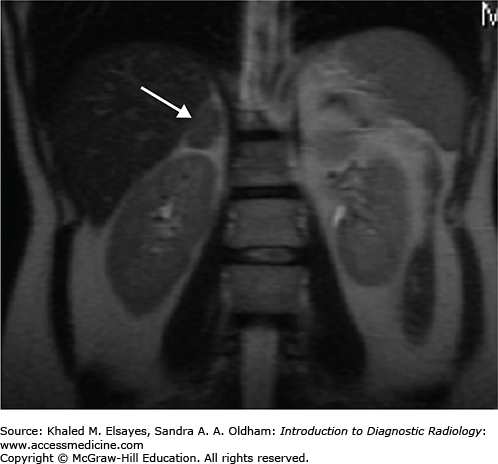
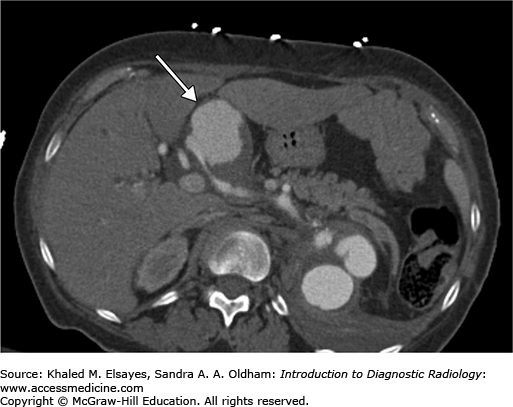
An MRI of the abdomen shows a 2-cm right adrenal adenoma (Fig. C3.1).
Medical management
Surgical excision
Adrenal vein sampling is performed prior to surgical excision in a patient with an elevated venous blood aldosterone-renin ratio. A diagnosis of primary hyperaldosteronism due to an adrenal adenoma in a symptomatic patient is necessary prior to adrenalectomy.
Absolute contraindications include an unstable patient or coagulopathy. Relative contraindications include renal insufficiency or severe contrast allergy.
The goal of adrenal vein sampling is to determine whether there is autonomous secretion of aldosterone from the adrenal adenoma. In these cases, the tumor can be excised surgically. In some cases, there may be increased secretion bilaterally due to bilateral adrenal cortical hyperplasia, which is managed medically. Samples taken from bilateral adrenal glands compare the aldosterone and cortisol levels from each adrenal gland versus systemic levels (Fig. C3.2). This can help direct the treatment or rule out adrenal gland hypersecretion as a culprit for the patient’s symptoms.
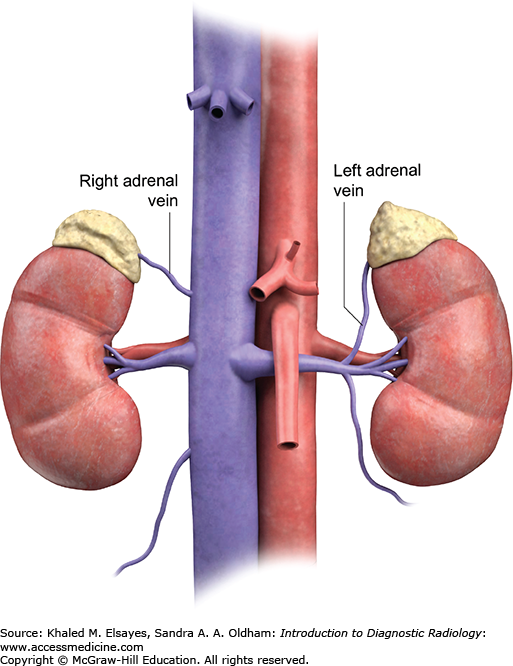
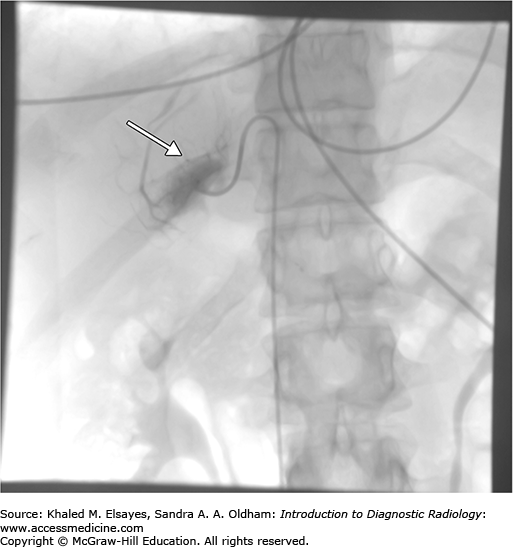
Typically, access to the inferior vena cava is achieved via the right femoral vein after cleansing of the skin and local anesthesia of groin with lidocaine. A 5 Fr curved catheter is advanced through a femoral vein access. The right adrenal central draining vein is usually located in the mid posterior IVC above the right renal vein. The left adrenal vein drains into the left renal vein. Contrast injection is used to confirm cannulation of the adrenal vein, which demonstrates a delta or triangular pattern of the adrenal gland (Fig. C3.3). Gentle suction or passive bleeding is then used to obtain blood sample for assay. A peripheral vein sample is also simultaneously obtained to compare the cortisol levels, which should be significantly lower in the peripheral vein than the central adrenal vein.
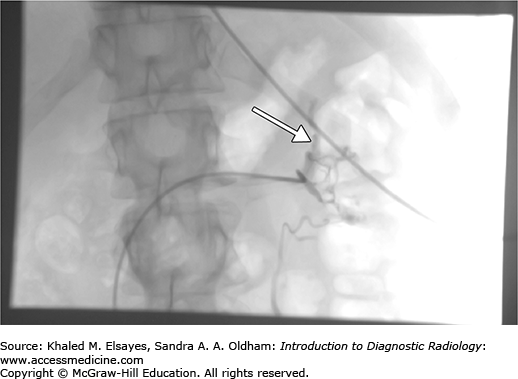
The left adrenal vein usually drains into the left renal vein. Using a reverse curve catheter, the left adrenal central draining vein is accessed via the left renal vein and samples are obtained (Fig. C3.4).
Adrenal vein rupture
Dissection
Thrombosis
Adrenal gland hemorrhage or infarction
Hypertensive crisis
Groin or retroperitoneal hemorrhage
Infection
CASE 4: ANEURYSM COIL EMBOLIZATION
A 70-year-old male with right upper quadrant pain.
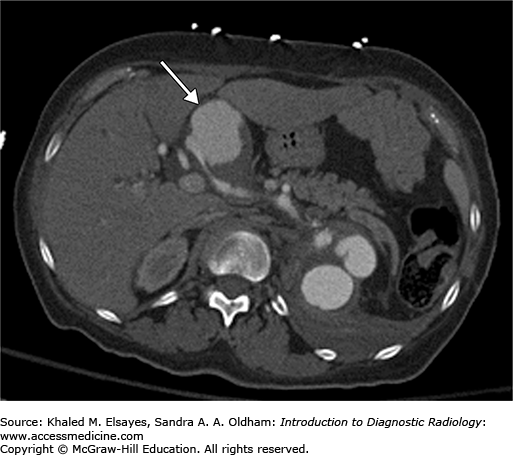
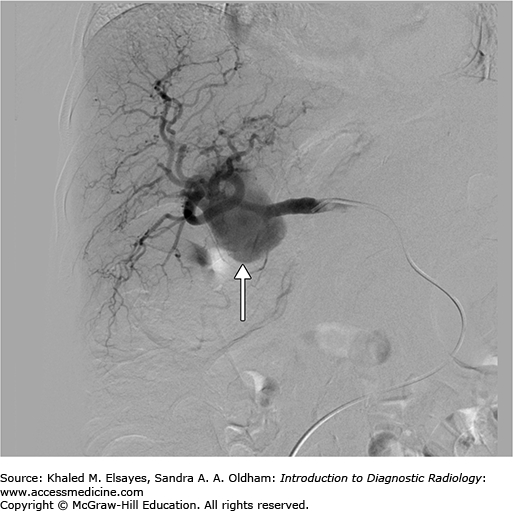
A CT of the abdomen demonstrates a large hepatic artery aneurysm (Fig. C4.1).
Medical management
Surgical ligation
Endovascular coil embolization
Symptomatic aneurysms are always treated. There is controversy over treatment of asymptomatic aneurysms. Generally, solid organ or splanchnic arterial pseudoaneurysms or enlarging true aneurysms are treated due to an increased chance of rupture. Hepatic artery aneurysms are treated preemptively due to increased risk of rupture in women who are pregnant or plan to become pregnant, patients with portal hypertension, or liver transplant candidates.
There are no absolute contraindications to embolization of hepatic artery aneurysm, especially in cases of rupture and hemorrhage.
Relative contraindications include:
Severe allergy to intravenous contrast
Renal insufficiency
Coagulopathy
Pregnancy
The goal is to occlude the aneurysm to prevent hemodynamic forces from enlarging it further or causing it to rupture. This can be achieved by placing a covered stentgraft across the aneurysm neck, embolizing the proximal and distal arteries, or embolizing the aneurysm with multiple coils.
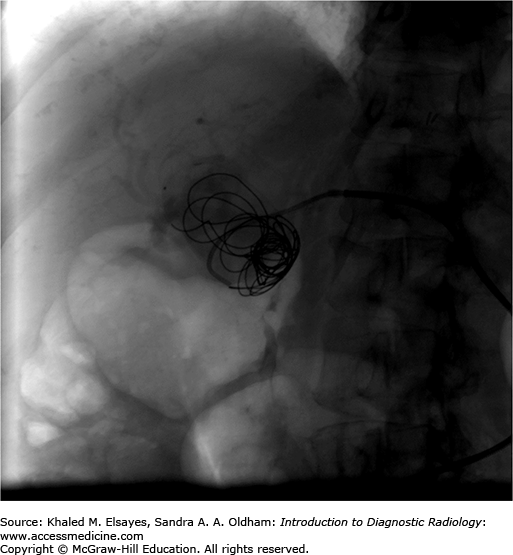
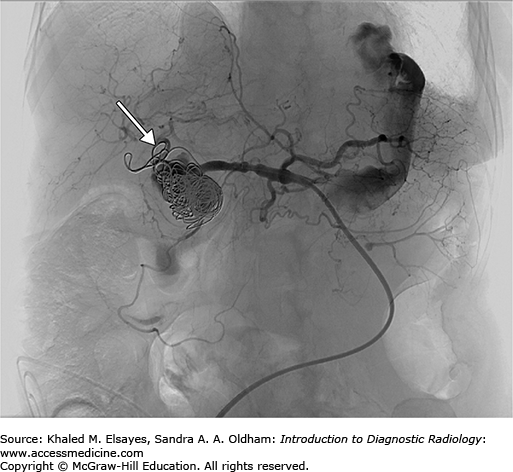
Access to the hepatic artery is typically achieved via the femoral artery or brachial artery approach. Typically, a 5 Fr curved catheter is use to gain a stable access to the celiac axis. An angiogram is performed to map the arterial anatomy (Fig. C4.2). Once the common hepatic artery is identified, the 5 Fr catheter or a microcatheter can be advanced to the hepatic artery aneurysm neck and multiple coils are advanced into the aneurysm using a guidewire (Figs. C4.3 and C4.4). In other cases, based on the arterial anatomy and size of the artery, a stent graft can be placed across the neck of the aneurysm or coils can be used to occlude the proximal or distal feeding vessels to stop the flow of blood into it. Confirmation of complete occlusion is identified via another angiogram.
Hemorrhage
Hepatocyte or biliary infarction
Pseudoaneurysm
Artery dissection
Infection
Nontargeted embolization resulting in infarction of visceral organs
CASE 5: ARTERIOVENOUS FISTULA DECLOTTING
A 35-year-old male with renal failure and a malfunctioning arteriovenous fistula.
A Doppler ultrasound shows arteriovenous (AV) fistula thrombi with diminished velocities.
Surgical thrombectomy
Endovascular thrombectomy/thrombolysis
Evaluation of the arteriovenous fistula is warranted when it fails to mature, malfunctions, or completely fails.
Absolute contraindications include uncorrectable coagulopathy and either systemic or fistula infections. Relative contraindications include:
Contrast allergy.
Significant cardiopulmonary disease that may be complicated by pulmonary emboli.
Ischemia distal to the arteriovenous anastomosis that may worsen following increased flow through the fistula.
The goal of declotting is to restore high flow through the fistula that is optimal for hemodialysis.
Ultrasound is used to evaluate the location of thrombus and the fistula is accessed using a needle pointing toward the anastomosis and another pointing away from the anastomosis. These are then upsized to 6 or 7 Fr sheaths. An angiogram is performed to confirm forward flow in the fistula, which is essential to prevent arterial emboli (Fig. C5.1). A guidewire is passed across the thrombus. Mechanical thrombectomy can then be done using angioplasty and a sweeping motion with a Fogarty balloon (Fig. C5.2), spinning a catheter or using a mechanical thrombectomy device such as a trerotola device. Chemical thrombolysis can also be done with tPA (tissue plasminogen activator) injected directly into the clot at the time of the procedure or over a period of 1 to 2 days using an infusion catheter. Follow-up angiogram is performed down to the right atrium to confirm declotting.
Chemical thrombolysis can also be used to soften clots less than 5 days old, followed by mechanical thrombectomy.
Occasionally, venous or anastomotic stenoses may be encountered. Every stenosis greater than 50% is potentially hemodynamically significant. A combination of balloon angioplasty and stents can be used to dilate the stenosis to less than 30%, and the flow is then documented on subsequent angiogram.
Venous rupture leading to nonfunctioning fistula
Sepsis
Bleeding
Pulmonary emboli
Pseudoaneurysm formation
CASE 6: PERCUTANEOUS BILIARY DRAINAGE CATHETER
A 45-year-old male with elevated bilirubin, jaundice, and abdominal pain.
CT scan of the abdomen and pelvis shows a pancreatic head mass obstructing the common bile duct resulting in biliary dilatation.
Whipple procedure with hepaticojejunostomy
ERCP with biliary stent placement
Percutaneous biliary drainage catheter
Decompression of the biliary system in patients with biliary obstruction from unresectable tumors, biliary stricture, liver transplant, or primary sclerosing cholangitis. Patients with cholangitis needing biliary diversion prior to surgery or brachytherapy for treatment of cholangiocarcinoma are also appropriate candidates.
Relative contraindications include thrombocytopenia and elevated INR.
Percutaneous biliary drainage catheter placement can be performed under ultrasound and fluoroscopy guidance.
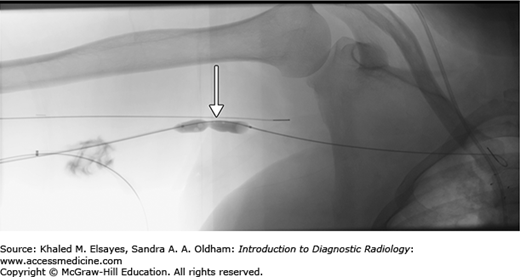
Ultrasound and fluoroscopic guidance are fast and effective in placement of percutaneous biliary catheters. The patient is placed in supine position. The overlying skin is cleansed and anesthetized. Using sterile technique, a 20-gauge needle is directed into the liver to access the bile ducts. If the ducts are dilated, ultrasound can be used to directly puncture one of the dilated hepatic ducts. Alternatively, under fluoroscopic guidance the needle can be directed toward the hepatic hilum from the liver periphery under fluoroscopy. Contrast is injected as the needle is advanced to opacify any hepatic ducts that may be encountered (Figs. C6.1 and C6.2). This method includes trial and error. It is not unusual to puncture the hepatic artery/vein or portal vein while looking for the bile ducts. Alternatively, the needle is advanced into a central position and then withdrawn while aspirating for bile or injecting contrast. Once the needle punctures bile ducts, a microwire is advanced into the bile duct (Fig. C6.3). A sheath with stiffener is advanced over the wire and confirmation of appropriate placement is done with contrast injection. The cholangiogram maps the biliary anatomy, confirms the point of obstruction, and evaluates communication of the right and left hepatic ducts. The sheath allows a stiffer wire to be advanced into the bile duct to the point of obstruction.
Fig. C6.1
Right transhepatic needle placement into a dilated right hepatic duct (arrow). Contrast injection shows appropriate access of the biliary system.