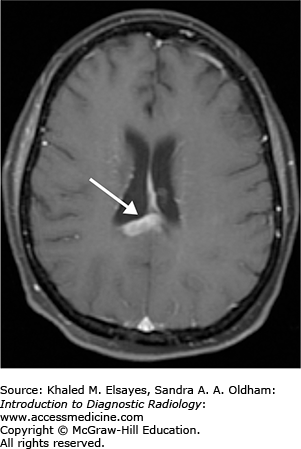
ANATOMY
This section serves as an introduction to the basic anatomy of the brain, head, neck, and spine. Brain anatomy is complex, and a complete discussion is beyond the scope of this text.
The human brain can be roughly divided into the cerebrum, thalamus, brain stem, and cerebellum. The cerebrum is comprised of the cerebral cortex, basal ganglia, and limbic system. The brain stem includes the midbrain, pons, and medulla. The cerebellum is composed of the cerebellar hemispheres and vermis.
Figures 3.1 to 3.10 illustrate the structures and blood vessels of the brain, head, neck, and spine.
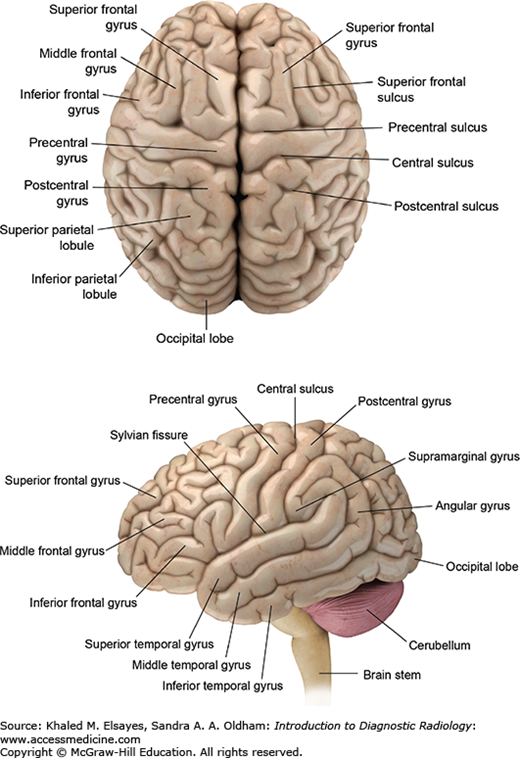
Fig. 3.1
Surface anatomy of the brain. The cerebral cortex is composed of gyri (ridges) and sulci (intervening depressions) divided into 4 lobes: frontal, parietal, temporal, and occipital. The frontal lobe is separated from the parietal lobe by the central sulcus. The sylvian fissure separates the temporal lobe from the more superiorly located frontal and parietal lobes. The occipital lobe is separated from the parietal lobe by the parieto-occipital sulcus.
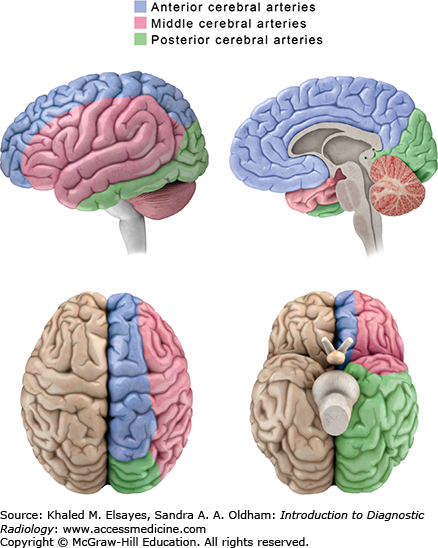
Fig. 3.2
Vascular distribution of the brain parenchyma. An understanding of the territories of the major intracranial vessels is important in the diagnosis of stroke and other vascular lesions. The anterior cerebral arteries (blue) supply the anterior parasagittal regions. The middle cerebral arteries (pink) supply most of the lateral cortex of the frontal and superior temporal lobes. The posterior cerebral arteries (green) supply the inferior temporal and occipital lobes.
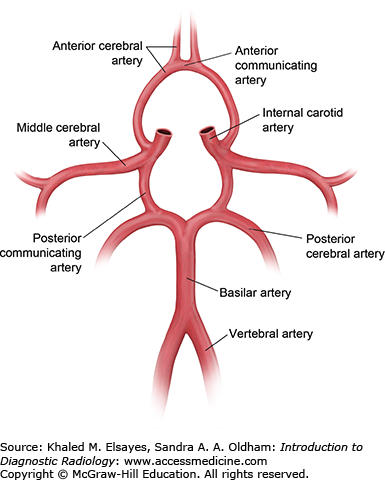
Fig. 3.3
Circle of Willis. The circle of Willis describes a loop of arteries that supply most of the brain. The posterior circulation consists of the 2 vertebral arteries that join to form the midline basilar artery. The basilar artery terminates into paired posterior cerebral arteries. The anterior circulation is predominantly supplied by the internal carotid arteries that terminate into the anterior and middle cerebral arteries. The anterior communicating artery connects both the anterior cerebral arteries, while the posterior communicating arteries connect the anterior and posterior circulation. The circle of Willis plays an important role in providing collateral supply in the setting of arterial occlusions and strokes.
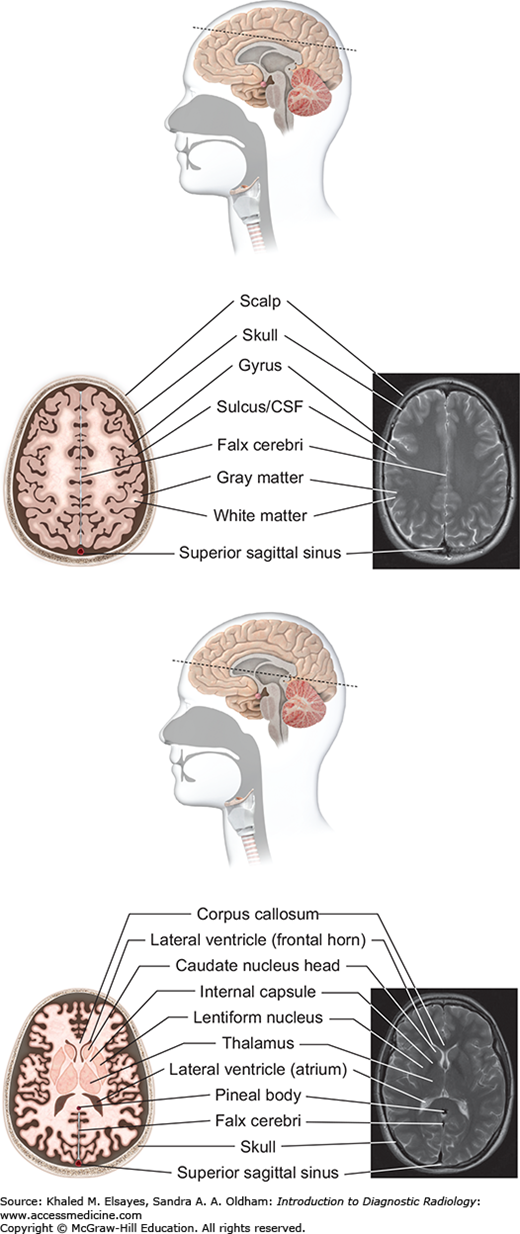
Fig. 3.4
Supraventricular level (top): At the supraventricular level, the frontal and parietal lobes are seen in cross section. The falx cerebri is a dural reflection that separates the 2 hemispheres. The superior sagittal sinus is seen posteriorly. Lateral ventricular level (bottom): The lateral ventricles are seen on this level as paired frontal horns anteriorly and the atria more posteriorly. The basal ganglia are seen, as are the thalami.
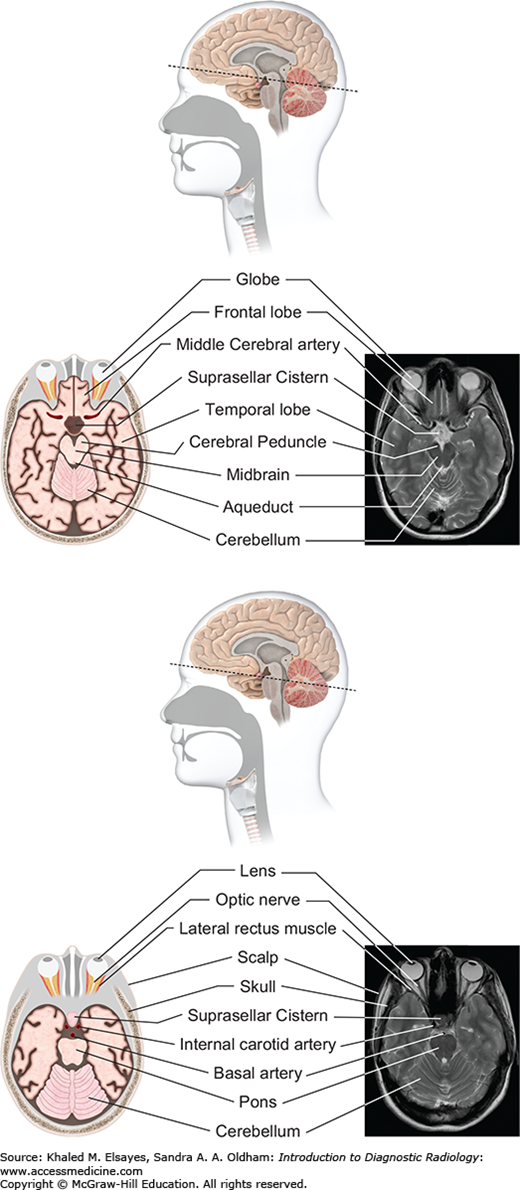
Fig. 3.5
Midbrain level (top): At the level of the midbrain, the paired temporal lobes are seen, between which lies the midbrain. The suprasellar cistern (a cerebrospinal fluid [CSF]–filled space at the base of the brain) is seen in the midline. The midbrain is composed of the tectum, tegmentum, and cerebral penducles. The cerebral aqueduct connects the third ventricle with the fourth ventricle. Pons level (bottom): At this level the pons is seen, along with inferior temporal lobes and the upper cerebellum. The pons contains several white matter bundles that connect the cerebrum to the cerebellum and medulla and also contains several cranial nerve nuclei.
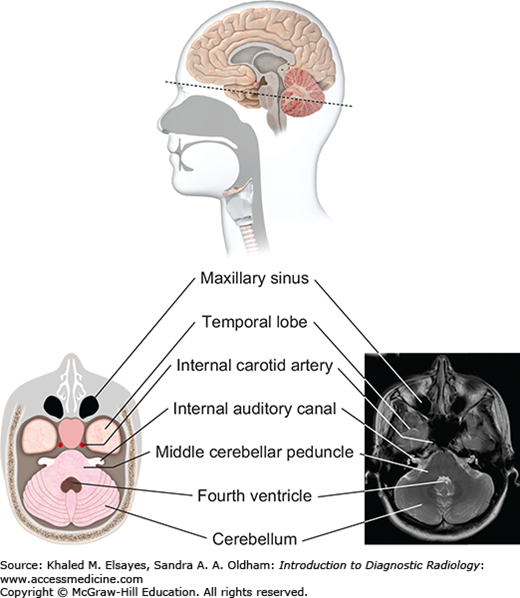
Fig. 3.7
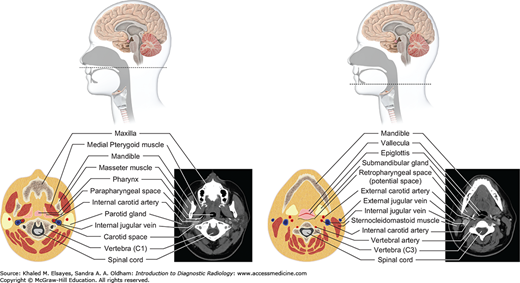
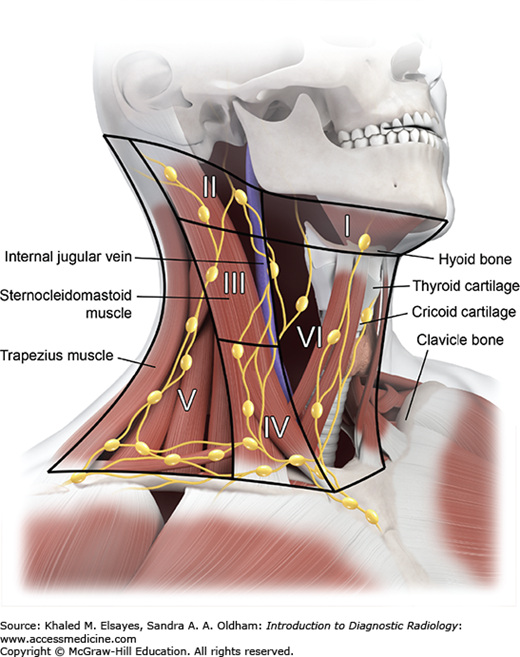
Suprahyoid neck (top and bottom): The suprahyoid neck (above the hyoid bone) contains the pharynx centrally. The paired fat-containing parapharyngeal spaces are seen adjacent to the pharynx. Anterolateral to the parapharyngeal space lies the masticator space, while posterolaterally lies the carotid space. The retropharyngeal and prevertebral spaces lie posterior to the pharynx.
Fig. 3.8
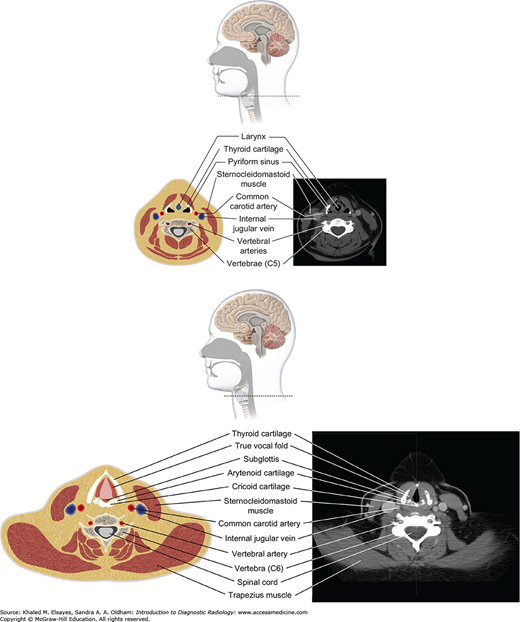
Infrahyoid neck (top and bottom): Below the hyoid bone lie the larynx and hypopharynx. The larynx is surrounded by several cartilaginous structures: thyroid cartilage, cricoid cartilage, arytenoid cartilage, and cricoid cartilage. The vocal cords are situated within the central larynx and divide the larynx into the supraglottic and subglottic spaces. The hypopharynx consists of the piriform sinuses, postcricoid region, and posterior pharyngeal wall.
Fig. 3.10
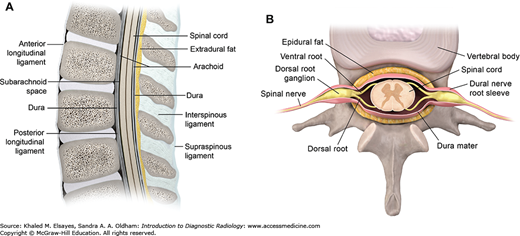
(A) Sagittal diagram of the spine, (B) axial diagram of the spine. The vertebral column contains vertebral bodies, intervertebral disks, and posterior elements. The spinal canal bounded by posterior elements contains the spinal cord, exiting nerve roots, surrounding cerebrospinal fluid, dura, and epidural fat/vessels.
INTRODUCTION TO BRAIN MR SEQUENCES
MR (magnetic resonance) is the cornerstone of modern imaging of the brain and spinal cord. Images are obtained by taking advantage of resonance properties of nuclei under the influence of an external magnetic field. Some basic sequences that the reader should become familiar with are shown in Figs. 3.11 to 3.13.
Fig. 3.11
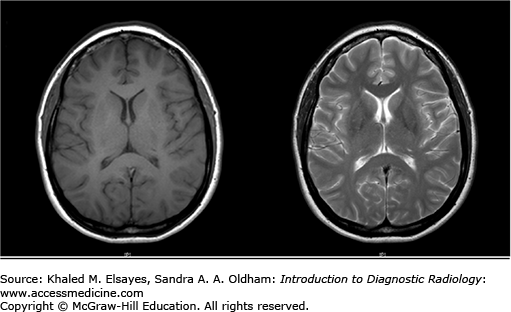
On T1-weighted imaging (left), white matter is bright due to the presence of myelin and gray matter structures including the cortical gray matter appear darker. Note that simple fluid such as CSF is black. Fat, subacute hemorrhage, melanin, and mineralization can appear bright on T1-weighted images. On T2-weighted imaging (right), CSF is bright, and white matter is dark. On both pulse sequences, there is loss of signal from flowing blood referred to as a flow void.
Fig. 3.12
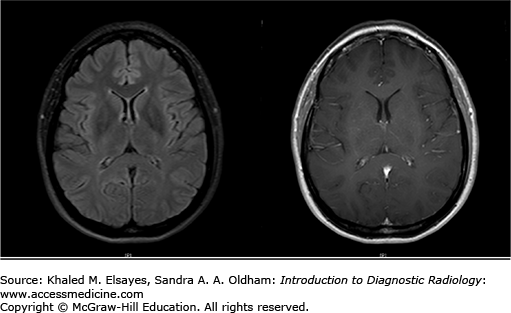
FLAIR imaging (left) is very similar to T2-weighted imaging in which white matter is relatively hypointense; however, the signal from CSF is suppressed. This sequence is helpful as any pathological process resulting in vasogenic or cytotoxic edema is hyperintense. Postcontrast images (right) are T1-weighted sequences obtained after the administration of gadolinium-based contrast agents. The vessels appear bright due to the presence of contrast. Postcontrast images are useful in detection of infectious, inflammatory, and neoplastic processes.
CASE 1
A 72-year-old male presents to the emergency department with acute onset of focal sensory loss and right arm weakness.
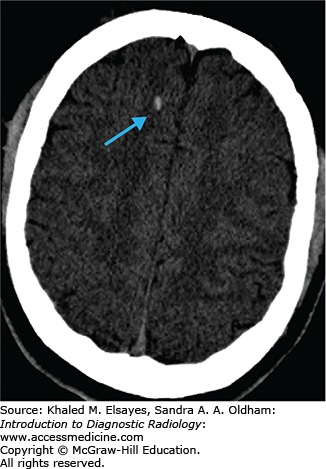
Acute ischemic stroke
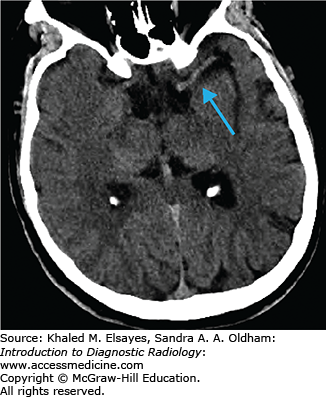
Noncontrast CT of the head to evaluate for hemorrhage and early ischemic changes. Advantages: fast and readily available, high sensitivity for presence of hemorrhage.
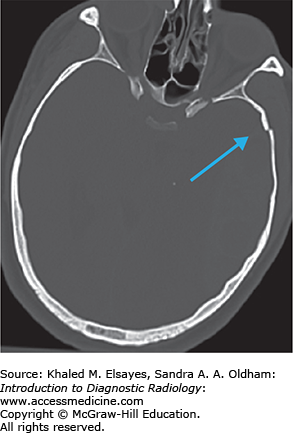
Cerebral infarcts are the leading cause of disability and fourth leading cause of death in the United States. Patients with acute cerebral ischemia/infarct usually present with sudden-onset neurologic deficit (weakness, sensory loss, aphasia). Risk factors include diabetes, hypertension, smoking, and hyperlipidemia. It is important to realize that the value in CT is not to diagnose an acute ischemic infarct. The role of CT is to exclude hemorrhage, exclude nonischemic pathologies (masses, infections, vascular malformations), and evaluate the volume of infarcted territory. In the setting of cerebral infarcts, the infarcted territory results in contralateral symptomatology.
Usually normal in the hyperacute phase
Hyperdense vessel representing acute thrombus (low sensitivity, high specificity) (Fig. C1.1)
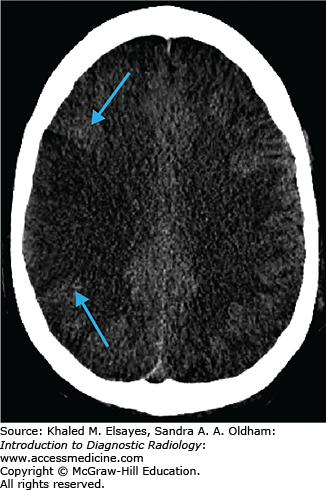
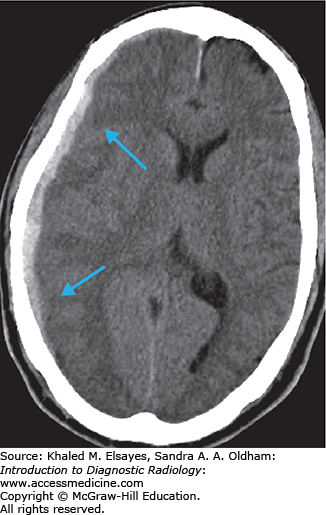
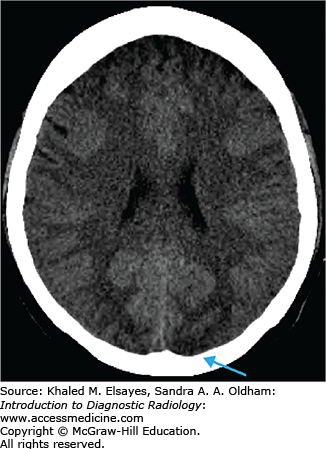
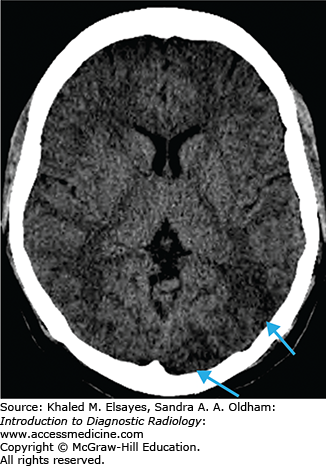
Loss of gray matter–white matter differentiation
“Insular ribbon” sign: swelling and blurring of normal gray-white differentiation within the insular cortex
Obscured lentiform nucleus
Parenchymal hypodensity resulting from early cytotoxic edema (Fig. C1.2)
Gyral swelling with sulcal effacement
Additional CT examinations that may be performed:
CT angiography: useful in localizing area of arterial stenosis or occlusion
CT perfusion: useful in identification of penumbra (brain at risk), which may help stratify patients for therapeutic intervention
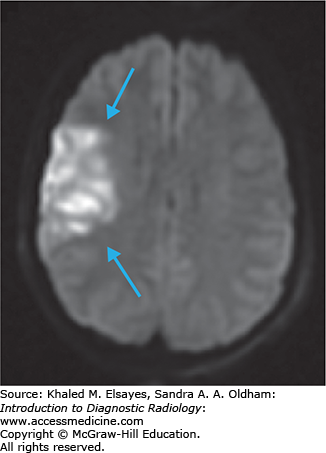
Highly sensitive to diagnose acute infarcts within minutes of onset.
Areas of acute ischemia restrict free diffusion of water, resulting in elevated signal on DWI images and decreased ADC values (Fig. C1.3).
Limitations of MRI in the emergency setting include difficulty to transport and monitor unstable patients, longer scan times, limited availability, and contraindications in certain patients (pacemakers, intraocular metallic foreign bodies).
Intravenous treatment of acute ischemic stroke with r-TPA can be considered if it is less than 4.5 hours since symptom onset, there is no CT evidence of hemorrhage, and the infarct does not involve more than one-third of the MCA distribution. Intra-arterial treatment with r-TPA can be considered up to 6 hours after symptom onset.
Acute hemorrhagic infarct
Neoplasm
Encephalitis
Todd’s paralysis
Hyperdense vessel: high hematocrit, atherosclerosis.
Parenchymal hypodensity: infiltrating neoplasm, encephalitis/cerebritis, encephalomalacia, dural venous thrombosis with venous congestion and edema.
CASE 2
A 59-year-old hypertensive male presents to the emergency department with headache and acute sensory deficit.
Hypertensive hemorrhagic stroke
CT head without contrast to evaluate for presence of hemorrhage (Fig. C2.1). Advantages: fast and readily available, high sensitivity for presence of hemorrhage.
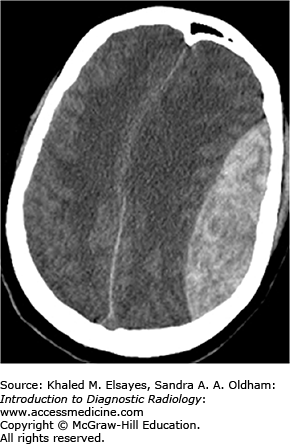
Chronic hypertension results in damage to small perforating arteries leading to fibrinoid necrosis and vessel fragility. Hemorrhagic strokes account for only 10% of all strokes (the majority are ischemic) and are associated with a higher mortality rate. Patients with hypertension have a fourfold increased risk of intracranial hemorrhage. The most common locations include the basal ganglia/external capsule, thalamus, brain stem, and cerebellum; however, approximately 10% are lobar. Initially, there may be little mass effect or surrounding edema; however, this often develops in the first couple of days leading to patient deterioration. As treatment differs markedly from ischemic stroke, prompt diagnosis is essential.
Round or oval hyperdense lesion in the brain parenchyma on noncontrast CT with predilection for certain locations as described earlier.
Other evidence of hypertensive cerebrovascular disease including chronic lacunar infarcts, multiple subacute/chronic microbleeds, and microvascular white matter changes.
Hemorrhage on MR has varied imaging characteristics (Table C2.1, Figs. C2.2 and C2.3):
Characteristics of Hemorrhage on MR
| Age of Hemorrhage | Component | T1 | T2 |
| Hyperacute | Oxyhemoglobin | Isointense/hypointense | Hyperintense |
| Acute | Deoxyhemoglobin | Isointense/hypointense | Hypointense |
| Subacute | Methemoglobin | Hyperintense | Hyperintense |
| Chronic | Hemosiderin/ferritin | Hypointense | Hypointense |
Gradient echo sequences may show several hypointense foci secondary to microbleeds. It is important to note that this is also commonly seen in cerebral amyloid angiopathy (disorder of vascular amyloid deposition leading to multiple bleeds).
No/mild peripheral contrast enhancement.
Ischemic stroke
Neoplasm
Encephalitis/meningitis
Hemorrhagic transformation of an ischemic infarct
Cerebral amyloid angiopathy
Underlying neoplasm or vascular lesion
Cocaine abuse
Coagulopathy
Venous thrombosis
CASE 3
Patient presents to the emergency department after hitting his head in a car accident.
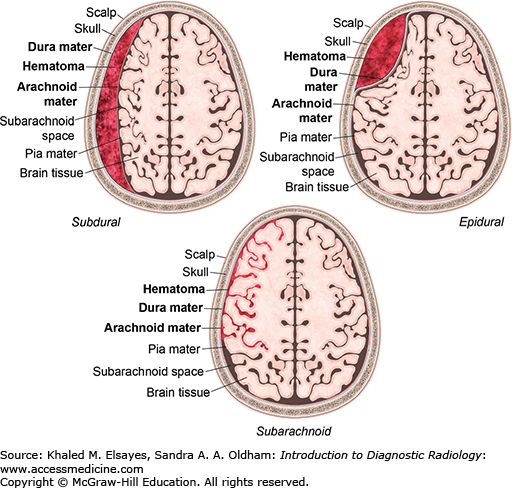
Traumatic intracranial extra-axial hemorrhage
CT head without contrast. Advantages: fast and readily available, high sensitivity for presence of hemorrhage.
Injuries involving the central nervous system are the leading cause of death in trauma patients. Intracranial hemorrhage is often seen in patients with moderate to severe head trauma. Acute hemorrhage is usually hyperdense on noncontrast CT images. In some instances the hemorrhage may have low density due to severe anemia, DIC, and hyperacute active bleeding, making its detection more difficult. There are several types of extra-axial hemorrhage based on location, as shown in Fig. C3.1.
The epidural space is a potential space between the inner table of the skull and the dura mater. CT shows a hyperdense biconvex extra-axial collection (Figs. C3.2 and C3.3). It does not cross suture lines due to firm attachment of the dura to the suture but may cross the midline. Bleeding most commonly results from tearing of the middle meningeal artery from calvarial fracture, secondary to trauma. Most epidural hematomas are found in the temporal and parietal regions. They may also be found in the posterior fossa secondary to a venous sinus tear. Approximately half of patients will have a lucid interval after the insult.
Collection of blood in the space between the dura mater and the arachnoid layer. CT shows a hyperdense crescentic extra-axial collection (Fig. C3.4). It can cross suture lines but does not cross the midline. Bleeding results from tearing of bridging cortical veins as a result of rotational motion of the brain with respect to the fixed venous structures. This most commonly occurs over the cerebral convexities. Subdural hematomas are often secondary to trauma but can occur secondary to minor trauma in elderly patients and patients on anticoagulation.
CT shows hyperdensity in the subarachnoid space (Fig. C3.5); 4 different patterns may be seen.
Adjacent to intraparenchymal or extraparenchymal hematomas: common in severe head trauma.
Overlying the cerebral hemispheres in isolation: common in mild and moderate head trauma.
Extensive hemorrhage within the basal cisterns and subarachnoid space: often seen in the setting of nontraumatic subarachnoid hemorrhage from ruptured aneurysm or arteriovenous malformation (AVM). It can also occur from traumatic arterial dissection, sometimes with concomitant skull base fracture.
Nontraumatic perimesencephalic hemorrhage: focal small hemorrhage in the interpeduncular cistern can be seen in patients presenting with headache without aneurysm. It usually results in no long-term clinical sequelae.
It is critical to evaluate patients with SAH for an intracranial aneurysm, regardless of a history of head trauma. A ruptured aneurysm may have been the precipitating event prior to the incident leading to the head trauma.
Epidural hemorrhage, subdural hemorrhage, subarachnoid hemorrhage, diffuse axonal injury (DAI), and concussion.
Epidural hemorrhage: subdural hemorhhage, abscess, tumor, inflammatory lesion, extramedullary hematopoiesis.
Subdural hemorrhage: EDH, subdural hygroma, pachymeningitis (thickened dura), tumor.
Subarachnoid hemorrhage: exudates in the subarachnoid space, serpentine calcifications from cortical laminar necrosis, arteriovenous malformation, Sturge-Weber syndrome, racemous neurocysticercosis.
CASE 4
An unconscious trauma patient presents to the emergency department and has a normal noncontrast head CT.
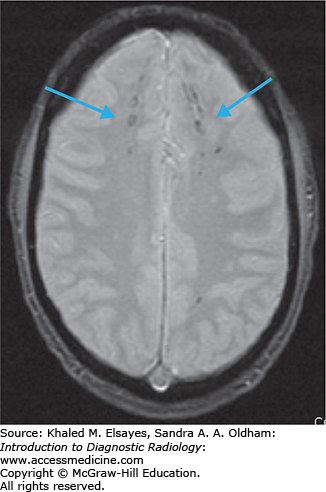
Diffuse axonal injury (traumatic brain injury)
MRI brain without contrast. Advantages: excellent contrast between normal and edematous brain parenchyma, highly sensitive for small hemorrhages due to susceptibility of blood products.
Diffuse axonal injury (DAI) is a shearing/stress injury caused by rotational acceleration/deceleration movements of the head. Brain tissues of different densities accelerate/decelerate at varying speeds, and particularly in regions where these tissues intersect the axons get stretched, resulting in damage. Small white matter vessels may also tear, resulting in petechial hemorrhages. Injuries are most commonly nonhemorrhagic, but hemorrhagic lesions also occur. Initial noncontrast CT is usually normal.
Multiple bilateral punctate to 1.5 cm round or ovoid foci with the long axis parallel to fiber bundles.
Nonhemorrhagic foci: bright on T2-weighted and FLAIR sequences.
Hemorrhagic foci: bright on T1, dark on T2, bright on FLAIR, and very dark on gradient echo images (due to susceptibility from blood products). GRE is the most sensitive sequence.
DWI may show areas of restricted diffusion (bright signal).
Lesions are found in characteristic locations with increasing order of injury severity:
Gray-white matter junction, typically in the frontal and temporal lobes, and cerebral white matter (Figs. C4.1 and C4.2).
Corpus callosum, most common in the splenium.
Dorsal upper brain stem and cerebellum.
Additional less common locations: deep gray matter, internal/external capsule, tegmentum, and fornix. Lesion burden on MRI may predict the degree of potential neurological recovery.
Additional MRI sequences can be performed such as diffusion tensor imaging and MR spectroscopy, which can be helpful in cases in which conventional MR imaging is inconclusive.
May show punctate areas of high density (hemorrhage) surrounded by a collar of low-density edema.
Usually underestimates the extent of the disease.
DAI should be considered if the patient’s symptoms are disproportionate to the imaging findings. DAI carries a poor prognosis, and patients may remain in a persistent vegetative state.
EDH, SDH, SAH
Multifocal nonhemorrhagic lesions: age-related lesions (leukoaraiosis and lacunes), demyelinating disease, Marchiafava-Bignami syndrome (corpus callosum lesions).
Multifocal hemorrhagic lesions: cerebral amyloid angiopathy, chronic hypertension, cavernous malformations, hemorrhagic metastases.
CASE 5
A 24-year-old pregnant female presents to her physician with elevated blood pressure and sudden onset of headache and visual disturbance.
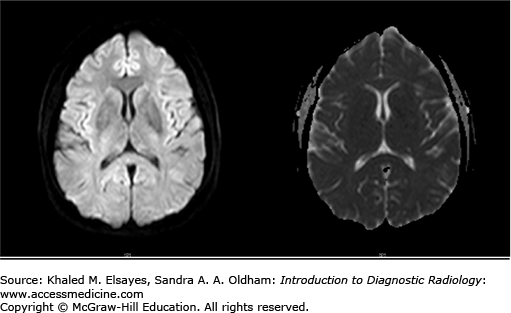
Posterior reversible encephalopathy syndrome (PRES)
MRI head with and without contrast (include DWI). Advantages: T2/FLAIR images are sensitive for edema.
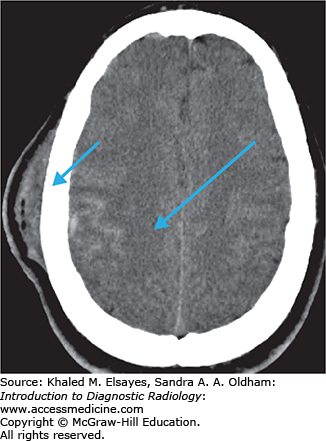
Bilateral, symmetric, patchy abnormalities classically involving the subcortical white matter of the occipital and parietal lobes. It may also extend to the cortex and involve the temporal and frontal lobes, pons, and cerebellum.
Hypodensity in the subcortical white matter of the occipital and parietal lobes (Figs. C5.1 and C5.2).
T2-weighted image/FLAIR: high signal intensity in the affected areas.
T1-weighted image: corresponding low signal intensity.
DWI-ADC map: areas of DWI abnormality without ADC correlation are more common, but irreversible ischemia (high DWI signal with decreased ADC signal) may occur.
Enhancement can be seen but is not a common finding.
MR perfusion: may show decreased relative cerebral blood volume, decreased cerebral blood flow, and increased mean transit time.
Areas of hemorrhage are seen in approximately 15% of patients.
May demonstrate a vasculopathy type pattern with areas of vessel irregularity, consistent with vasoconstriction/vasodilation.
The underlying etiology of PRES is thought to be the inability of the posterior circulation to autoregulate in response to acute changes in blood pressure. This results in vascular damage and breakdown of the blood-brain barrier leading to vasogenic edema. Symptoms include headache, confusion, seizures, and loss of vision. Interestingly, hypertension is absent in approximately 25% of patients with PRES, alluding to alternative underlying mechanisms of the disease. With prompt diagnosis and treatment of the underlying cause, PRES is usually reversible; in some instances it may progress to infarction.
PRES may be caused by hypertension, preeclampsia/eclampsia, immunosuppressive drugs, chemotherapy (tacrolimus and cyclosporine), sepsis, renal disease, autoimmune diseases, cryoglobulinemia, and hemolytic-uremic syndrome.
Primary headache/migraine
Preeclampsia/eclampsia not associated with PRES
Venous sinus thrombosis
Intraparenchymal hemorrhage
Acute ischemic infarct
Status epilepticus
Metabolic abnormalities
Gliomatosis cerebr
CASE 6
A 28-year-old female presents with sudden onset of severe headache, confusion, and right extremity weakness.
Intracranial hemorrhage, possibly from vascular malformation
CT head without contrast in the acute setting followed by CTA or MRA. Definitive diagnosis/characterization performed with catheter angiography. Advantages: fast and readily available, high sensitivity for presence of hemorrhage.
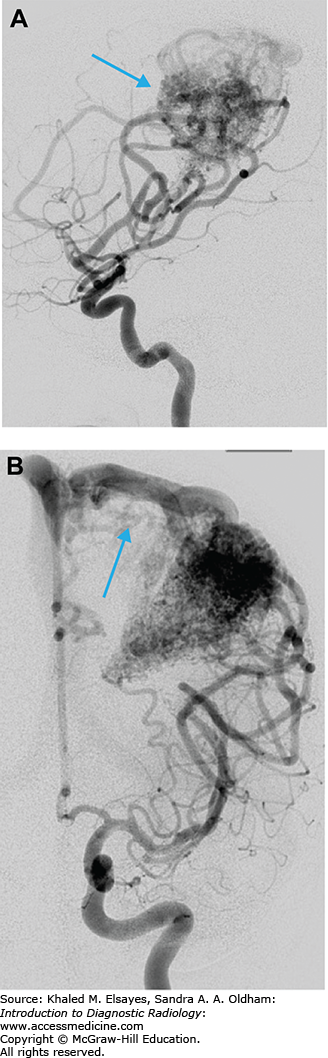
Arteriovenous malformations are abnormal connections/networks of blood vessels that result in arteriovenous shunting of blood, bypassing blood supply to the brain tissue (Fig. C6.1A,B). They are characterized by an enlarged feeding artery/arteries leading to a nidus of entangled abnormal vessels (no true capillary bed) and early drainage into enlarged draining veins (Fig. C6.2). In the acute setting, noncontrast head CT is the imaging modality of choice.
Intraparenchymal hematoma if ruptured. Can also result in intraventricular or subarachnoid hemorrhage.
If unruptured, hyperdense serpentine mass lesion.
Curvilinear or speckled calcifications may be seen.
Surrounding hypodensity of gliosis (chronic ischemia or previous hemorrhage).
Intensely enhancing curvilinear structures on CTA, curvilinear flow voids on MRI.
Dilated feeding arteries and draining veins; veins typically larger than arteries.
Intranidal aneurysms (found more than 50% of the time).
Catheter angiography provides dynamic information showing the arterial supply and venous drainage of the lesion.
Pitfalls in diagnosis can occur with small lesions, location near a major dural venous sinus, and due to compression from acute hematoma (repeat angiogram is warranted if initial study is normal).
AVMs can be classified using the Spetzler-Martin grading system in order to predict operative outcomes as shown in Table C6.1 (graded 1 to 5 by adding the points in each category).
Grades 1 and 2 are treated with surgical resection; Grade 3 lesions receive multimodality treatment (microsurgery/endovascular/radiosurgery); Grades 4 and 5 are not treated, with the exception of recurrent hemorrhages, progressive neurological deficits, steal-related symptoms, and AVM-related aneurysms. Imaging features that are associated with a risk of future hemorrhage include prior hemorrhage (low signal intensity on gradient echo sequence), intranidal aneurysms, venous stasis or ectasia, deep venous drainage, single venous drainage, and deep or posterior fossa locations. Imaging features associated with risk of nonhemorrhagic neurological deficits include high-flow shunt, venous congestion or outflow obstruction, long pial course of draining vein, perifocal or perinidal gliosis, mass effect or hydrocephalus, and arterial steal.
CVA, underlying neoplasm, amyloid angiopathy
Hypertensive bleed, other vascular lesions (cavernous hemangioma), intratumoral hemorrhage, dural sinus thrombosis with hemorrhagic infarct, mycotic aneurysm, drug abuse.
CASE 7
A 53-year-old female with history of hypertension presenting with complaints of intermittent headache. Patient recalls having been diagnosed with benign tumor many years ago.
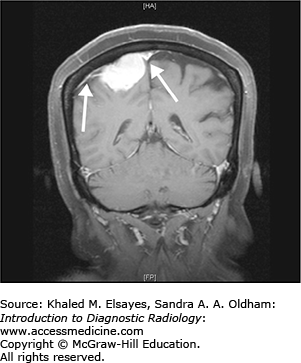
Meningioma
In the acute setting, noncontrast CT is obtained to exclude intracranial hemorrhage. MRI is preferred for evaluation of intracranial neoplasms as it provides greater sensitivity, superior parenchymal detail, and better tissue contrast.
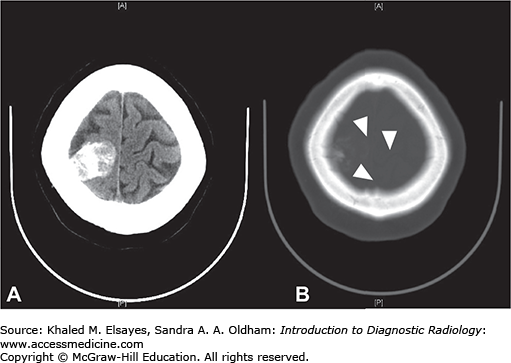
Meningiomas are tumors that arise from the meninges and as a result are extra-axial. While most meningiomas are benign (WHO Grade 1), atypical (WHO Grade 2) and malignant meningiomas (WHO Grade 3) can rarely be seen.
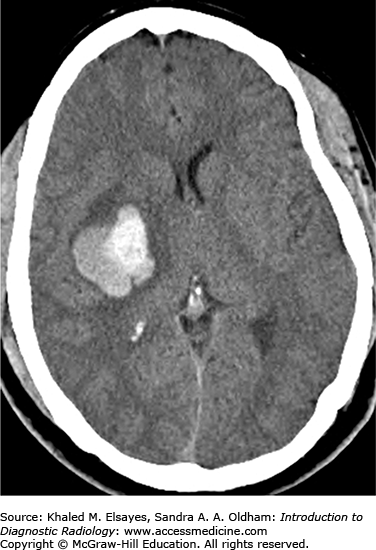
Hyperdense extra-axial mass on noncontrast CT (Fig. C7.1)
20% to 25% are calcified
Hyperostosis (thickening) of the adjacent calvarium
Avidly enhance, with enhancement of a dural tail
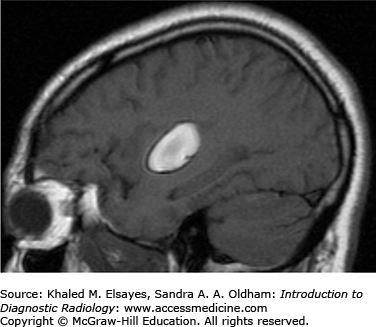
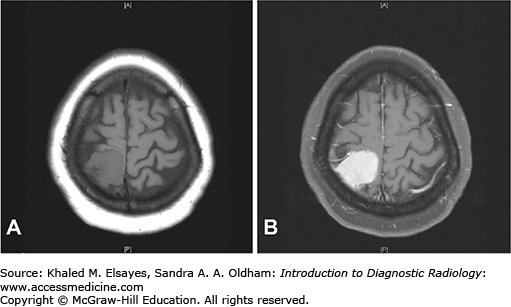
T1: isointense to hypointense to cerebral cortex; may be heterogeneous due to cystic degeneration and/or hemorrhage (Fig. C7.2).
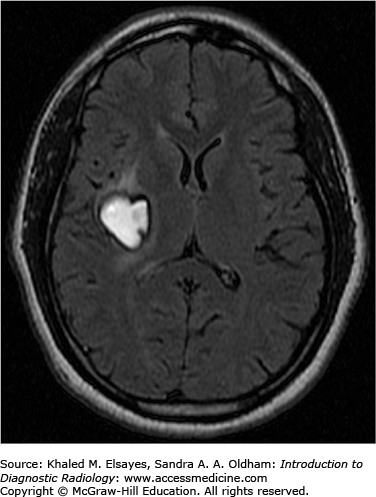
T2: variable, large lesions can be associated with edema within the subjacent brain parenchyma; visualization of a CSF cleft between mass and adjacent brain parenchyma is helpful in diagnosis of an extra-axial mass.
T1+C: avid enhancement with enhancement of a dural tail (Fig. C7.3).
While most patients with typical meningioma are asymptomatic, patients with larger lesions may present with varied symptoms based upon the location of the lesion. Parasagittal lesions may present with seizures and weakness; basisphenoid/diaphragm sella lesions with visual defects, and cavernous sinus lesions with cranial nerve palsies.
Metastasis: Dural-based metastasis can mimic meningiomas; however, patients usually have a known primary tumor and may have other sites of metastatic disease.
Granuloma: Meningeal involvement from sarcoid or TB can mimic meningioma; however, these patients often have signs/symptoms of disease elsewhere in the body.
Lymphoma: Extra-axial areas of lymphomatous involvement are usually multifocal and present in patients with known history of lymphoma. Lesions demonstrate hypointensity on T2 images due to hypercellularity.
Hemangiopericytoma: Can mimic meningioma; however, calcification and hyperostosis are usually absent. Enhancement is usually more heterogeneous.
CASE 8
A 48-year-old male presents to his physician with new-onset seizures.
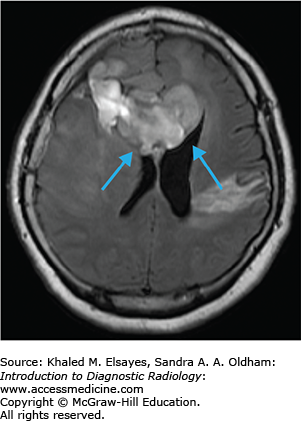
High-grade glioma / glioblastoma multiforme (GBM)
MRI brain with and without contrast. Advantages: MRI is preferred for evaluation of intracranial neoplasms as it provides greater sensitivity, superior parenchymal detail, and better tissue contrast. Diffusion weighted imaging, perfusion imaging, and MR spectroscopy may be helpful in evaluation of intracranial neoplasms.
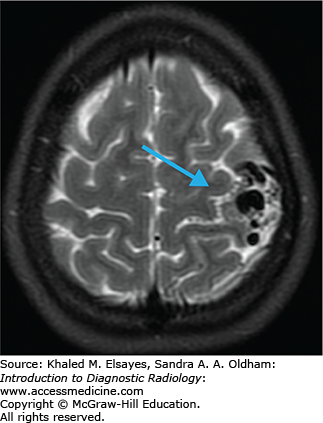
While most intra-axial neoplasms are metastatic, glioblastoma multiforme (WHO grade 4 tumor) accounts for approximately 25% of all intra-axial neoplasms. It is a highly malignant infiltrating astrocytoma with a poor prognosis (10%-15% two-year survival). These tumors can occur anywhere but most commonly arise, in decreasing order of frequency, in the temporal, parietal, and frontal lobes. GBMs can either be a primary tumor (arising de novo) or a secondary tumor (resulting from dedifferentiation of a lower grade astrocytoma).
T1: usually heterogeneously isointense to hypointense. Heterogeneity results from internal necrosis; some cases present with hemorrhage.
T2/FLAIR: heterogneously hyperintense with surrounding edema/infiltrative tumor (Fig. C8.1).
T1+C: variable. Typically there is an irregular rind of enhancement surrounding a central area of necrosis (Fig. C8.2).
The tumor frequently crosses the midline to the contralateral hemisphere by direct extension through the corpus callosum, anterior commisure, or posterior commisure (“butterfly” tumor). It is important to realize that microscopic infiltration of tumor beyond the borders of imaging abnormality. Increased relative cerebral blood volume (rCBV) can be seen on perfusion studies and has been shown to correlate with the histological grade (higher rCBV = higher histological grade). The presence of lactic acid on MR spectroscopy and the presence of intratumoral hemorrhage have also been linked to higher-grade tumors.
Medication/drug related seizures, CVA, metabolic disorder, meningitis/encephalitis, posttraumatic, autoimmune/degenerative diseases, neoplasm.
Metastasis.
Ring-enhancing lesions: abscess, demyelinating disease, resolving hematoma, subacute cerebral infarction, radiation necrosis.
Corpus callosum lesions: lymphoma, multiple sclerosis.
CASE 9
Patient presents to the emergency department with seizure and has a history of AIDS.
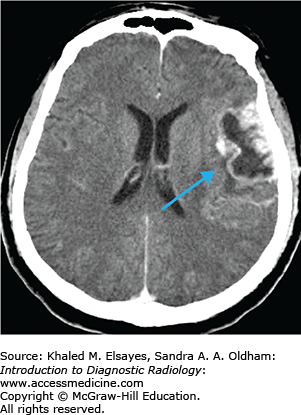
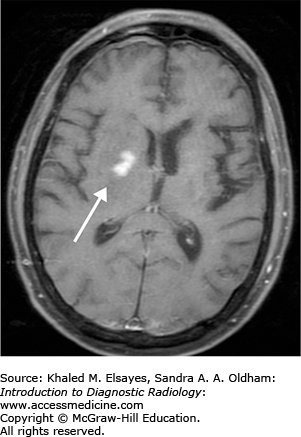
Opportunistic infection versus neoplasm
MRI brain with and without contrast. Advantages: MRI is preferred for evaluation of intracranial infections or neoplasms as it provides greater sensitivity, superior parenchymal detail, and better tissue contrast.
The vast majority of primary CNS lymphoma is non-Hodgkins lymphoma and usually occurs in immunocompromised individuals. These lesions are mostly supratentorial in location (above the tentorium cerebelli) and commonly involve the periventricular white matter and deep gray matter. CNS lymphoma may spread along the ventricular lining (subependymal) and cross the midline. It usually presents with multiple lesions that are less than 2 cm in size. Primary CNS lymphoma in immunocompetent patients is less often seen and usually has a later onset.
High-density mass/masses typically periventricular in location on noncontrast exam with variable enhancement.
Central necrosis may be present; hemorrhage rarely present.
T1: isointense or hypointense lesions (Fig. C9.1).
T2/FLAIR: variable; approximately half will be isointense or slightly hypointense owing to tumor cellularity. Increased T2/FLAIR signal surrounding lesions representing edema.
T1+C: variable, usually solid or ring enhancement (Fig. C9.2).
DWI: mildly restricted diffusion due to high cellularity.
An alternative diagnosis found in AIDS patients that must be differentiated from lymphoma (due to similar imaging characteristics) is toxoplasmosis (Table C9.1). Toxoplasmosis is the most common cause of focal lesions in AIDS patients.
Lymphoma Versus Toxoplasmosis in AIDS
| Characteristic | Lymphoma | Toxoplasmosis |
| Present with solitary lesion | 19% | 39% |
| Lesion size | 53% 1-3 cm | 52% <1 cm |
| Solid enhancement | 76% if <1 cm, 50% if >1 cm | 77% if <1 cm, 23% if >1 cm |
| Ring enhancement | 24% if <1 cm, 50% if >1 cm | 23% if <1 cm, 77% if >1 cm |
| Dense on noncontrast CT | 33% of lesions | None |
| T2-weighted image | 55% isointense | Usually hyperintense or mixed |
| Periventricular/subependymal/corpus callosum location | 25% of pts/38%/3% | 3% of pts/none/none |
| Basal ganglia location | 7% of pts | 17% of pts |
| Thallium-201 SPECT scan | Avid uptake (>2cm lesions) | No uptake |
| MR perfusion | Increased rCBV | Decreased rCBV |
| MRS | Increased choline and lipid, low NAA | Increased lactate |
| Steroid/radiation therapy | Very sensitive | No response |
HIV encephalitis, other CNS infections (toxoplasmosis, CMV, PML, aspergillosis, cryptococcus, TB), alcohol withdrawal, drug toxicity, CVA, hemorrhage, metabolic disorders.
Diffuse/patchy WM abnormalities in AIDS: HIV encephalitis, PML, CMV.
Focal/multifocal brain lesions in AIDS: toxoplasmosis, lymphoma, tuberculoma, fungal, abscess.
CASE 10
A 5-year-old female presents to the clinic with several-week history of increasing ataxia and 3-day history of nausea/vomiting.
Brain tumor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree