INTRODUCTION
Imaging in the pediatric population is unique, and it is essential for physicians to limit patient exposure to ionizing radiation in the medical workup due to increased risk of cancer development. Children are at higher risk of cancer development because they are more radiosensitive and live more years than adults. The use of nonionizing radiation in children with ultrasound and MRI is recommended when possible. Also, as demonstrated in the case presentations in this chapter, the initial imaging modalities of choice in the evaluation of the majority of pediatric patients include radiographs, which have a low amount of ionizing radiation, and ultrasound. Therefore, it is important to be aware of the imaging modalities and techniques available to promote radiation protection in the imaging of children and to take a conscientious approach to imaging this population.
CASE 1: SURFACTANT DEFICIENCY DISEASE
Premature infant born at 28 weeks of gestation develops shortness of breath shortly after birth.
Surfactant deficiency disease
Chest radiograph
Surfactant deficiency disease, also called respiratory distress syndrome or hyaline membrane disease, is primarily seen in premature infants. It is a lung disease that occurs as a result of surfactant deficiency, resulting in alveolar collapse and decreased pulmonary compliance.
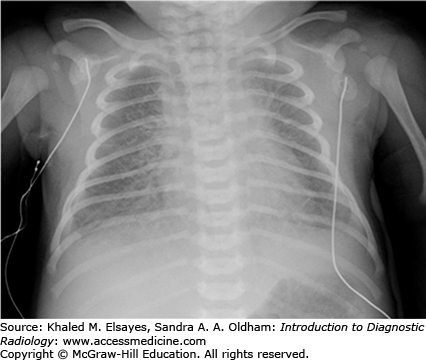
On frontal chest radiograph, there are low lung volumes associated with diffuse haziness or fine reticular granular opacities due to generalized alveolar collapse (Fig. C1.1). Prominent air bronchograms are seen due to patent larger bronchi in the diseased lung. Pleural effusions are an uncommon finding with surfactant deficiency disease.
Treatment with surfactant replacement therapy can lead to clearing of the granular opacities and increased lung volumes. In some cases, there may be asymmetric clearing of lung opacities indicating partial response to treatment. In addition to surfactant replacement therapy, neonates with surfactant deficiency commonly require mechanical ventilation for treatment and respiratory support.
During this time, chest radiograph may show coarse granular opacities and mixed areas of overinflation and atelectasis before returning to normal appearance. Appearance on the chest radiograph may mimic meconium aspiration leading to misdiagnosis if not clinically correlated. Mechanical ventilation can also be associated with several potential complications, including pulmonary interstitial emphysema and air-block complications such as pneumomediastinum, pneumothorax (Fig. C1.2), and pneumopericardium.
Acute pulmonary interstitial emphysema most often occurs in the setting of severe surfactant deficiency and is a result of airway overdistention and subsequent rupture of alveoli with air entering the adjacent pulmonary interstitium. Radiographically, pulmonary interstitial emphysema appears as multiple well-defined cystic or bubblelike lucencies that can be focal or diffuse and often resolve with appropriate timely management (Fig. C1.3). Rarely, pulmonary interstitial emphysema may persist and develop into a localized expansive, multicystic mass on chest radiograph.
Several other common causes of shortness of breath in newborn infants include meconium aspiration syndrome, transient tachypnea of the newborn, and pneumonia. Meconium aspiration syndrome is respiratory distress due to intrapartum or intrauterine aspiration of meconium in mostly postterm babies. Transient tachypnea of the newborn, also referred to as wet lung disease, is self-limited and results from delayed clearance of fluid from the lungs. Neonatal pneumonia can occur intrauterine, during birth, or thereafter and may be caused by a number of pathogens, most commonly due to beta-hemolytic streptococci.
Evaluation of a chest radiograph in a newborn demonstrating diffuse pulmonary disease should begin with assessment of the lung volumes and pulmonary opacities. Low lung volumes are more commonly seen in infants with surfactant deficiency disease and beta-hemolytic streptococcal pneumonia, whereas high lung volumes are more common in meconium aspiration syndrome, transient tachypnea of the newborn, and other types of neonatal pneumonia. The lung volumes with all of these entities, however, may be normal.
Findings of meconium aspiration on chest radiograph include patchy and asymmetric lung opacities. Transient tachypnea of the newborn may demonstrate a combination of airspace opacities, prominent interstitial lung markings, and pleural effusion (Fig. C1.4). Neonatal pneumonia, other than beta-hemolytic pneumonia, is characterized by asymmetric, patchy perihilar opacities and may or may not have pleural effusion. Distinguished from the other types of neonatal pneumonia, the findings of beta-hemolytic pneumonia can be similar to those of surfactant deficiency disease with diffuse fine granular opacities.
CASE 2: CONGENITAL DIAPHRAGMATIC HERNIA
Term infant presents at birth with severe respiratory distress. Physical examination demonstrates a scaphoid abdomen and auscultation of the lungs reveals poor air entry on the left.
Congenital diaphragmatic hernia
Frontal chest radiograph
Congenital diaphragmatic hernia is caused by a defect in the diaphragmatic musculature with herniation of the abdominal contents into the chest, resulting in underdevelopment of the lungs. The lung ipsilateral to the hernia is most commonly affected with hypoplastic changes due to long-standing mass effect from the hernia. The opposite lung may also be affected by mass effect, which results from shift of the mediastinum with subsequent compression. Pulmonary hypoplasia or development is a major factor in determining prognosis of these infants. Three basic types of congenital diaphragmatic hernia exist: (1) posterolateral Bochdalek hernia, (2) anterior Morgagni hernia, and (3) hiatus hernia. Bochdalek hernia is the most common congenital diaphragmatic hernia and most often occurs on the left side because the liver protects the right posterior Bochdalek foramen.
In the prenatal period, ultrasound has a high sensitivity in the detection of congenital diaphragmatic hernia. Ultrasound demonstrates bowel loops undergoing peristalsis in the chest and absence of the abdominal stomach bubble. Fetal MR imaging is useful for determining the degree of pulmonary hypoplasia, which can be useful for predicting neonatal survival.
Associated anomalies, including congenital heart disease, can occur in 25% to 50% of patients with congenital diaphragmatic hernia and contribute to the patient’s mortality. Because the bowel herniates into the left side of the thorax, there are varying degrees of left lung hypoplasia, a leading cause of morbidity and mortality in these children. Primary treatment includes respiratory support with mechanical ventilation followed by surgical repair.
Typical findings on chest radiograph in infants with a left-sided posterolateral congenital diaphragmatic hernia include air-filled or fluid-filled loops of bowel in the left hemithorax, mediastinal shift away from the hernia, and paucity of bowel gas in the scaphoid abdomen secondary to bowel loops herniating in the chest (Fig. C2.1). In some cases, abnormal position of support tubes may also be a clue to the diagnosis. Often, the combination of clinical scenario and appearance of the abnormal radiolucency on chest radiograph are diagnostic of diaphragmatic hernia.
Infants presenting at birth with severe respiratory distress may be the result of several clinical etiologies. This may be secondary to a diffuse pulmonary lung disease such as neonatal pneumonia and meconium aspiration syndrome, or lung anomalies.
Congenital pulmonary airway malformation (CPAM) and congenital lobar emphysema are included in the differential of localized lung radiolucencies seen in neonates.
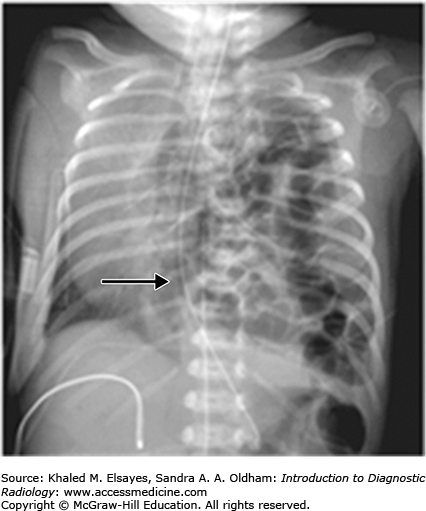
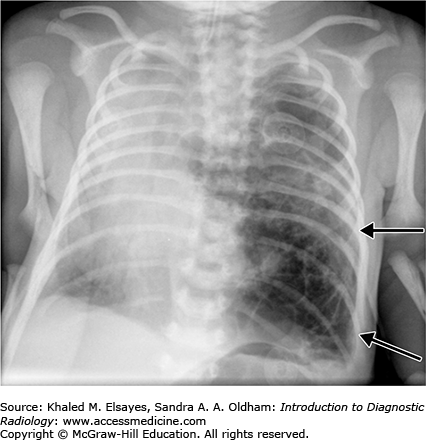
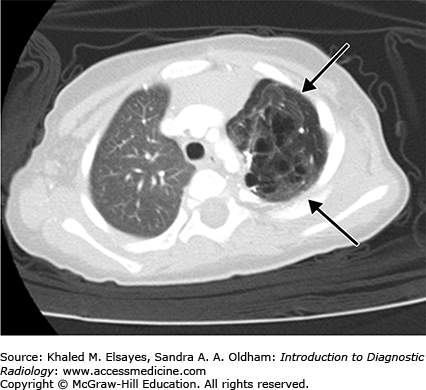
Congenital pulmonary airway malformation is a congenital adenomatoid proliferation of the bronchioles with lack of normal alveolar development (Figs. C2.2 and C2.3). A single lobe is typically involved and there is no lobar predilection. Three types of CPAMs exist, and they are differentiated based on the size of the cysts at imaging or pathology. Findings on chest radiography and CT differ with the type of malformation. The lesions may appear predominantly cystic, mixed solid and cystic, or solid depending on the size and number of cysts and whether the cysts contain air or fluid.
Congenital lobar emphysema is characterized by overinflation of the alveoli thought to result from a ball-valve type of anomaly in the bronchus that causes progressive air trapping. The most common lobe affected is the left upper lobe, followed by the middle lobe, right upper, and both lower lobes.
Chest radiographs and CT classically demonstrate a hyperinflation of the lobe involved. If seen in the immediate postneonatal period, however, it may mimic a solid masslike lobar lesion as it is completely filled with fluid. Gradually, it gets aerated with progressive although slow clearance of fluid, appearing partially cystic and partially solid; before it appears completely hyperinflated with air.
CASE 3: PNEUMONIA
A 5-year-old male presents with 3 days of fever and cough.
Pneumonia
Frontal and lateral chest radiographs should be obtained in children with suspected pneumonia.
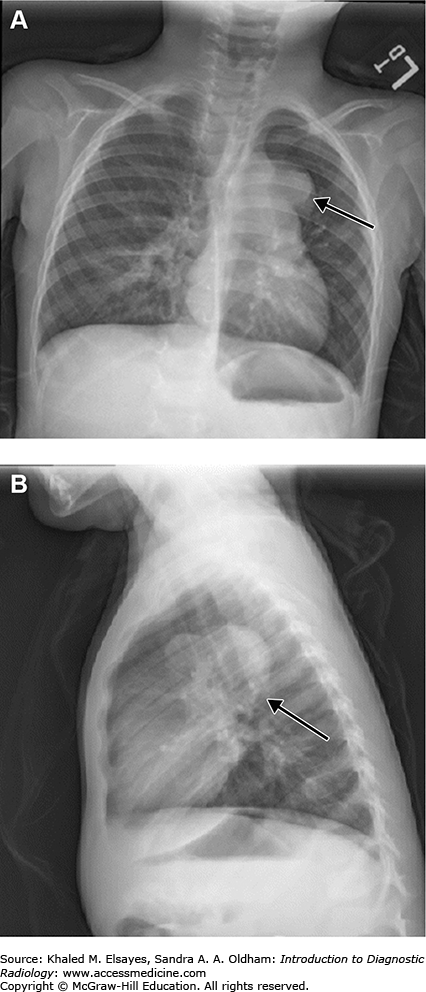
Evaluation of suspected community-acquired pneumonia is one of the most common indications for imaging in children. On chest radiograph, focal air space consolidation with air bronchograms in a lobar or segmental distribution or patchy areas of multifocal consolidation is seen (Fig. C3.1).
In younger children, bacterial pneumonia frequently has a very round, well-defined appearance on chest radiography, simulating a mass (Fig. C3.2). This is referred to as round pneumonia and is most common in children younger than 8 years of age. Round pneumonia is more common in the lower lobes and is most often caused by Streptococcus pneumoniae infection. The reason for this appearance in children is thought to be due to underdevelopment of collateral pathways of air circulation. In the appropriate clinical setting in a child with cough and fever, a lung mass seen on chest radiograph should be considered to be round pneumonia, and additional imaging with CT is not indicated. Follow-up radiographs should be obtained several weeks after appropriate antibiotic therapy to ensure resolution. After 8 years of age, however, if a round mass is seen on chest radiograph, other pathology should be considered.
Radiographs are frequently requested in pediatric patients with suspected pneumonia to help differentiate between bacterial and viral pneumonia. The cause of viral pneumonia in children varies depending on the child’s age, but viral infections are much more common than bacterial infections in all age groups. Viral infections cause inflammation of the small airways, which results in peribronchial edema and increased peribronchial markings on chest radiographs. These are seen as symmetric, ropelike linear markings radiating from the hila of the lungs. On the lateral view, the hila may appear prominent and the lungs will be hyperinflated (Fig. C3.3).
Common complications of pneumonia in children include parapneumonic effusion, empyema, cavitary necrosis, or lung abscess. Chest radiograph is the primary imaging modality for evaluation of these complications, though ultrasound or contrast-enhanced CT may be helpful in further evaluation if chest radiographs do not demonstrate any abnormalities.
Children with pneumonia most commonly present with cough, fever, and malaise, but may also present with abdominal pain. In the clinical evaluation of a child with cough and fever, differentiation between viral and bacterial infection is important in regard to treatment decisions. Also, consideration of other respiratory tract diseases such as croup, bronchiolitis, and bronchitis should be made.
There are several diagnoses that may appear similar to round pneumonia on chest radiograph. A bronchogenic cyst may appear as a round, well-defined soft tissue mass on a chest radiograph and can demonstrate a very similar appearance to round pneumonia (Figs. C3.4 and C3.5).
If the round pneumonia is posterior, it may simulate a posterior mediastinal mass such as neuroblastoma. But round pneumonia will have acute borders rather than obtuse borders with the mediastinum and neuroblastoma may demonstrate rib erosion or destruction. Other differential considerations include congenital pulmonary airway malformations or pulmonary sequestration.
CASE 4: EPIGLOTTITIS
A 3-year-old male presents with respiratory distress and drooling. Patient is sitting in a sniffing position and leaning forward with his head and nose tilted upward.
Epiglottitis
Lateral soft tissue neck radiograph
Epiglottitis is an acute life-threatening disease that is secondary to infectious inflammation of the epiglottis and surrounding soft tissues. This may result in airway obstruction and may potentially require emergent intubation. Haemophilus influenzae is the most common cause of epiglottitis, and since the vaccine for H influenzae has become available, the incidence of epiglottitis has significantly decreased. Children with epiglottitis usually develop sudden onset of symptoms and are toxic appearing with high fever, difficulty swallowing, drooling, and shallow breathing with the head held forward.
If the diagnosis is not made on physical examination, a single, lateral, upright view of the neck in extension is frequently diagnostic. Since there may be rapid progression of the condition leading to acute complete obstruction of the upper airway, radiographs should be obtained with minimal manipulation of the neck and with the patient in a comfortable position. Placing the patient supine to obtain a lateral neck radiograph may lead to acute airway obstruction and should be avoided. If the examination cannot be performed with portable equipment, a patient with suspected epiglottitis should be accompanied by someone with equipment readily available to intubate the child and secure the airway in the radiology department.
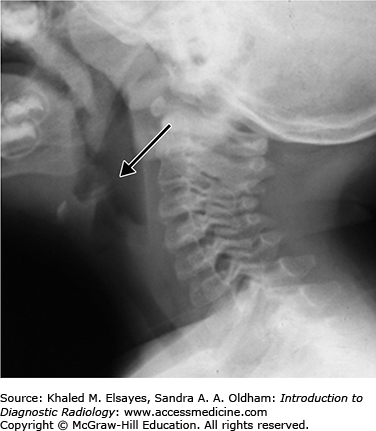
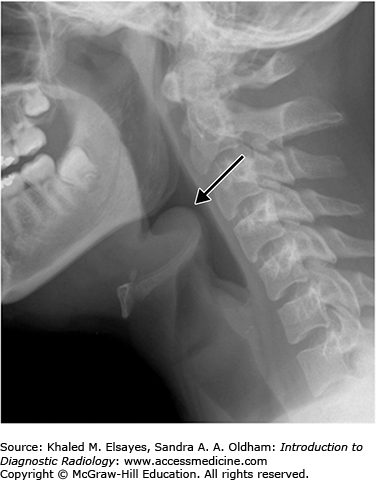
On lateral plain radiographs of the neck, the normal epiglottis is a thin, curved flap of soft-tissue opacity that is separated from the base of the tongue by air in the vallecula (Fig. C4.1). In epiglottitis, the epiglottis appears swollen and enlarged, with the so-called thumbprint sign, secondary to its appearance similar to a thumb (Fig. C4.2). Additional associated findings may include thickening of the aryepiglottic folds, prevertebral soft tissue swelling, and expansion of the hypopharynx.
The omega epiglottis is an epiglottis with prominent lateral folds and is a normal variant that should not be confused with a truly enlarged and swollen epiglottis. Also, if the epiglottis is imaged obliquely, it can appear falsely wide and thickened and may mimic findings of epiglottitis. In these cases, evaluation of the aryepiglottic folds, which remain thin and normal in appearance, may be helpful in making the distinction.
The most common clinical differential includes croup, bacterial tracheitis, and retropharyngeal abscess.
To help differentiate epiglottitis from croup, consider the age of the patient, prodrome, type of cough, and degree of toxicity. In general, croup occurs in younger children and has a viral prodrome. Children with croup also have a barking cough and rarely appear toxic, as in epiglottitis.
Bacterial tracheitis is characterized by infection of the trachea with exudative membranes on the tracheal wall. Affected children are ill and while uncommon, it is potentially life threatening if a membrane occludes the airway.
Retropharyngeal abscess generally occurs subsequent to upper respiratory tract infection or pharyngitis due to spread to the retropharyngeal lymph nodes.
As discussed earlier, it is important to differentiate between true epiglottitis and the omega variant or an epiglottis that is imaged obliquely and appears artifactually thickened.
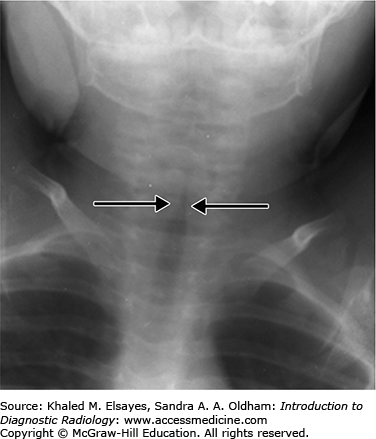
Findings of epiglottitis on a frontal radiograph may appear similar to the characteristic findings of croup. Croup causes symmetric subglottic narrowing, termed the steeple sign (Fig. C4.3).
Bacterial tracheitis demonstrates intraluminal tracheal membranes or tracheal irregularity on the lateral view of the neck.
Lateral radiograph showing thickening of the retropharyngeal soft tissues may represent retropharyngeal cellulitis or abscess. Visualization of gas on the lateral radiograph may confirm abscess formation, but it may be absent. CT evaluation of abscess demonstrates a rim-enhancing hypoattenuating area within the retropharyngeal soft tissues.
CASE 5: NECROTIZING ENTEROCOLITIS
Premature infant born after 26 weeks of gestation develops abdominal distention, feeding intolerance, and blood in the stool 2 weeks after birth.
Necrotizing enterocolitis
Abdominal radiograph
Necrotizing enterocolitis (NEC) is an idiopathic entity that is predominantly a disease of premature infants and most often occurs in those with extremely low birth weight of less than 1000 g. It generally manifests in the first 3 weeks of life and is likely the result of a combination of infection and ischemia. NEC may occur anywhere throughout the colon, although it most commonly involves the distal ileum and right colon. Symptoms include feeding intolerance, abdominal distention, sepsis, and bloody stool.
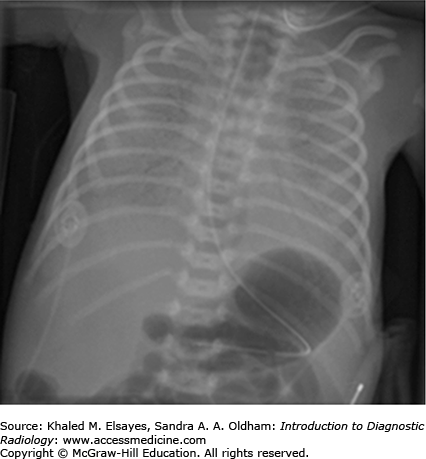
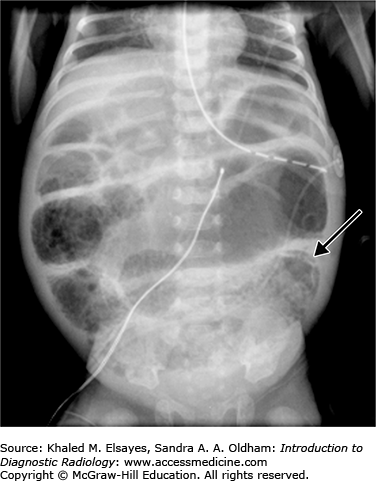
Early abdominal radiographic findings of necrotizing enterocolitis range from normal in appearance to findings of bowel distention, paucity of bowel gas in the right lower quadrant, or focal dilatation of bowel that does not change in appearance over time. As the disease process progresses, the presence of pneumatosis intestinalis, or gas within the bowel wall, is a definitive diagnostic finding. This is seen as bubblelike lucencies within the bowel wall mucosa (Fig. C5.1). Also diagnostic is the radiographic finding of portal venous air, which appears as branching linear lucencies overlying the liver.
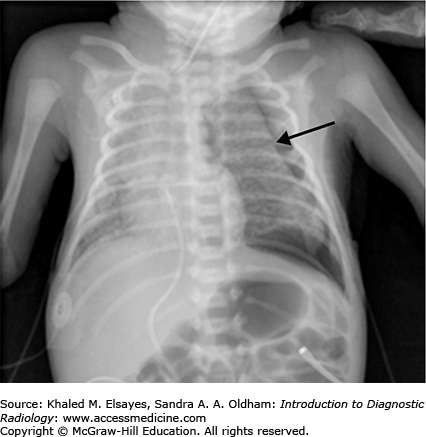
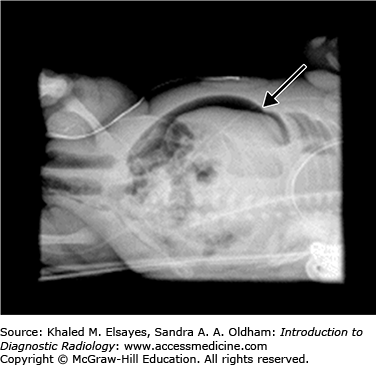
Free intraperitoneal air may be seen on abdominal radiographs when bowel perforation has occurred (Figs. C5.2 and C5.3) and is considered an absolute indication for surgical intervention. Multiple radiographic signs of free intraperitoneal air may be seen (refer to Table C5.1). In the absence of free air, treatment of NEC includes bowel rest, nasogastric decompression, and antibiotic therapy. The most common complication of NEC is bowel stricture.
Signs of Free Intraperitoneal Air
| 1. | An overall increased lucency of the abdomen on supine radiographs. |
| 2. | Visualization of both sides of the bowel wall, termed Rigler’s sign (Fig. C5.3). |
| 3. | Visualization of the outline of the intraperitoneal structures such as the falciform ligament, ligamentum teres, umbilical ligaments. |
| 4. | Large pneumoperitoneum outlining the entire abdominal cavity, termed the football sign (Fig. C5.3). |
| 5. | Pockets of anterior abdominal lucency on cross-table lateral radiographs. |
In cases of NEC in which the abdomen is distended and gasless, ultrasound may be beneficial. Gray-scale ultrasound may demonstrate thickened or dilated bowel loops and abdominal ascites. The bowel may have increased vascularity due to inflammation or decreased vascularity as a result of ischemia.
In premature infants demonstrating signs of abdominal distention and diarrhea, etiologies such as systemic and intestinal infections, congenital intestinal obstruction (eg, volvulus, pyloric stenosis, ileal atresia, Hirschsprung’s disease), and spontaneous bowel perforation should be considered.
The radiographic findings of necrotizing enterocolitis can range from normal to diagnostic. The differential diagnosis can include bowel obstruction or bowel perforation.
Bowel obstruction shows dilated loops of bowel that may include small bowel or large bowel loops depending on the site of obstruction. Air-fluid levels will be seen in the dilated bowel loops on upright abdominal radiographs.
In patients with bowel perforation, most commonly idiopathic, one or more of the previously discussed signs of free intraperitoneal air may be identified.
Other consideration includes nonspecific gaseous distention secondary to bag ventilation or intubation in appropriate clinical settings.
CASE 6: DUODENAL ATRESIA
Newborn male infant develops bilious emesis on the first day of life. No significant abdominal distention is present on physical examination.
The differential considerations for bilious emesis include multiple entities such as malrotation with midgut volvulus, duodenal atresia or other anomalies associated with intrinsic or extrinsic duodenum obstruction, and meconium ileus.
Abdominal radiograph is the initial imaging modality of choice. Duodenal obstruction may present as complete or partial obstruction depending on the etiology, but neonates showing evidence of complete duodenal obstruction at abdominal radiography rarely require further radiologic evaluation.
Duodenal atresia is caused by the failure of recanalization of the duodenal lumen during fetal development resulting in a blind ending duodenum and complete intrinsic obstruction. The duodenum is the most common site of intestinal atresia. Many conditions are associated with duodenal atresia including Down syndrome (approximately 30%), other intestinal atresias, congenital heart disease, imperforate anus, annular pancreas, and renal anomalies.
Infants with duodenal atresia most commonly present with vomiting within hours of birth. The majority of patients have bilious vomiting as the obstruction is mostly distal to the ampulla of Vater. Patients have feeding intolerance and there is usually little or no abdominal distention secondary to the proximal level of obstruction.
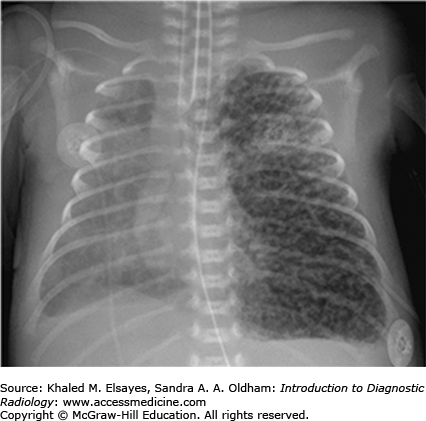
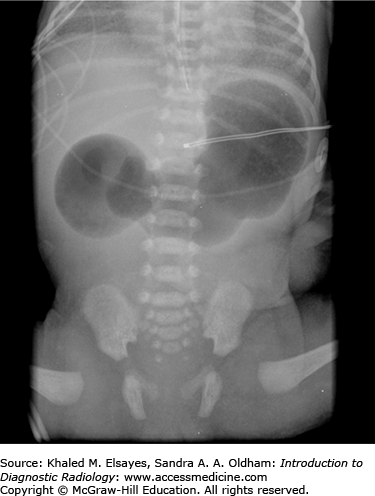
Abdominal radiographs in patients with duodenal atresia routinely demonstrate classic findings of a dilated stomach and a dilated proximal duodenum with no gas present distal to the proximal duodenum (Fig. C6.1). This is referred to as the “double-bubble” sign and is diagnostic of duodenal atresia given the appropriate clinical history. With these findings, further imaging with an upper GI series is not indicated, and there is potential hazard of vomiting with barium aspiration if an upper GI examination is performed.
Radiographic findings in patients with partial duodenal obstruction show gaseous distention of the stomach and duodenum with gas in the small bowel. Partial duodenal obstruction may be caused by duodenal stenosis, duodenal web, Ladd bands, malrotation with midgut volvulus, or annular pancreas. If distal bowel gas is present on abdominal radiographs, an upper GI series is indicated to evaluate and differentiate between the potential causes. In some cases, when there is a very small degree of obstruction, patients may remain asymptomatic or may be incidentally diagnosed during examination for another condition.
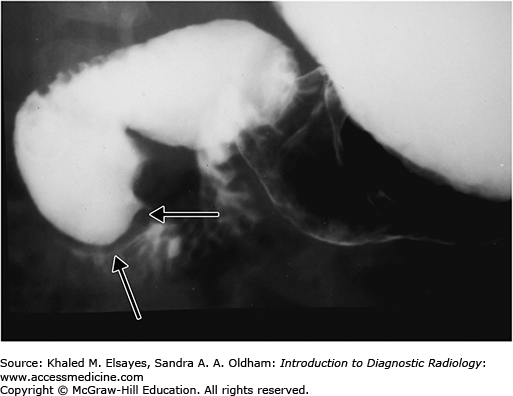
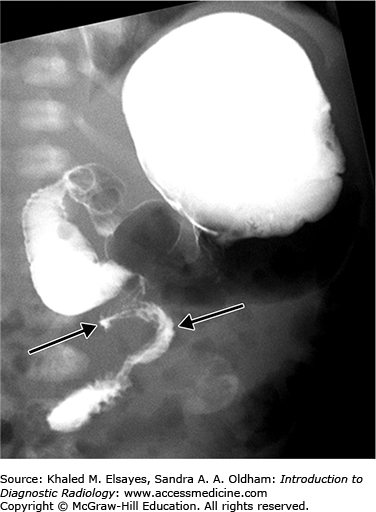
Duodenal stenosis and duodenal web are the result of partial canalization of the duodenal lumen during fetal development. A web is usually an obstructing membrane with a pinhole central lumen that is typically at or near the ampulla of Vater. On upper GI exam, duodenal web classically shows a windsock appearance within the duodenal lumen due to ballooning and stretching of the redundant membrane (Fig. C6.2).
Annular pancreas is an anomalous band of pancreatic tissue that encircles the second portion of the duodenum. Annular pancreas cannot be diagnosed at the time of an upper GI exam and may be diagnosed on CT or MRI, which demonstrate pancreatic tissue and an annular duct encircling the duodenum.
Intestinal malrotation is a congenital abnormal position of the bowel within the peritoneal cavity and applies to a wide range of intestinal anomalies. In patients with malrotation, there is abnormal fixation of the small bowel mesentery that results in a short mesenteric base that is prone to twisting. Malrotation of the bowel is associated with a number of syndromes and other anomalies, often occurring in association with other gastrointestinal abnormalities and heterotaxy syndromes. Volvulus due to malrotation occurs when there is twisting of small bowel around the superior mesenteric artery and vein that can result in bowel obstruction and bowel ischemia or necrosis.
Findings of malrotation and midgut volvulus on abdominal radiograph may be normal or may show distention of the stomach and proximal duodenum. A markedly dilated duodenal bulb may be seen in long-standing obstruction from midgut volvulus and may mimic the appearance of duodenal atresia, but will not be seen with acute volvulus. Radiographs may also demonstrate diffuse bowel distention, pneumatosis, portal venous gas, or free peritoneal air from bowel ischemia, necrosis, or perforation.
The upper GI series is the imaging modality of choice for the diagnosis of malrotation with or without volvulus. The diagnosis of malrotation is made by evaluation of the position of the duodenojejunal junction. The normal position of the duodenojejunal junction is to the left of the spine and at the same level as the duodenal bulb on frontal views and posterior, or retroperitoneal, on lateral views.
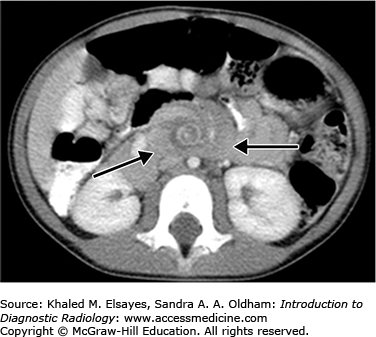
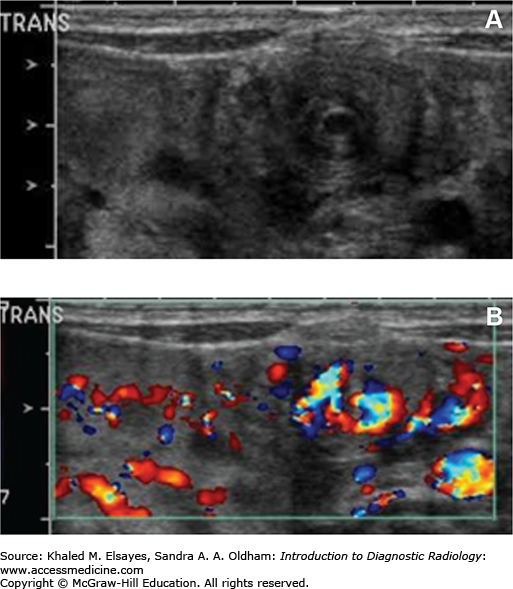
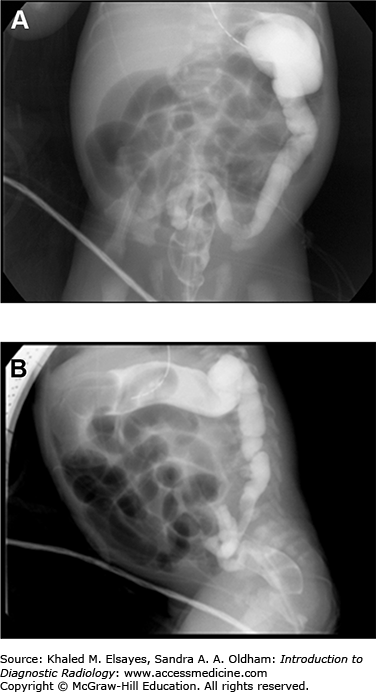
Patients with bowel malrotation have an abnormal position of the duodenojejunal junction on the upper GI series, and when midgut volvulus is present, the duodenum and proximal jejunum can be seen as Z shaped or corkscrew shaped (Fig. C6.3). CT and ultrasound in malrotation show similar findings with the superior mesenteric vein to the left of the superior mesenteric artery and volvulus indicated by a “swirl” or “whirlpool” sign of twisted mesenteric vessels and bowel (Figs. C6.4 and C6.5). On ultrasound, this is best visualized on color Doppler.
Differentiation among the causes of bilious emesis in newborns often cannot be made based on clinical history and physical examination. Radiologic evaluation beginning with abdominal radiograph is important in the workup and may help to provide pertinent information that can lead to the correct diagnosis.
The double bubble sign is the classic radiographic finding of duodenal atresia, but if gas is seen within the distal small bowel, other diagnoses as described earlier should be considered and an upper GI series should be performed to exclude malrotation with midgut volvulus, which is a potentially life-threatening complication.
CASE 7: HIRSCHSPRUNG’S DISEASE
Term male infant fails to pass meconium within the first 48 hours of life and has developed abdominal distention.
Hirschsprung’s disease
Abdominal radiograph is the initial imaging modality of choice. If findings on the initial radiographs are abnormal and suggestive of distal bowel obstruction, water-soluble contrast enema is the next step in the evaluation of the colon.
Hirschsprung’s disease is caused by an absence of ganglion cells within the colon that results in a functional obstruction secondary to spasm in the denervated colon. This condition accounts for approximately 15% to 20% of cases of neonatal bowel obstruction. The majority of patients with Hirschsprung’s disease present in the neonatal period with failure to pass meconium, but later presentation in childhood or later in life may occur with history of constipation and abdominal distention. Over 90% of cases present within the first 5 years of life, and a very small number may present as adults. Hirschsprung’s disease is more common in boys than girls (4:1), and an increased incidence is seen in patients with Down syndrome.
The disease can be classified based on the length of the aganglionic segment, always extending proximally from the anal canal. Short segment disease is most common and involves the rectum and distal sigmoid colon only.
Abdominal radiographs demonstrate findings of distal bowel obstruction including variable gaseous distention of the colon and small bowel, often with air-fluid levels. Performing a water-soluble/barium contrast enema may provide a definitive diagnosis and demonstration of a transition zone between the narrow and dilated portions of the colon.
The rectosigmoid ratio may be helpful in evaluation. In normal newborns, the rectum has a larger diameter than the sigmoid colon, giving a rectosigmoid ratio greater than 1. In Hirschsprung patients, however, the maximal diameter of the rectum is smaller than the maximal diameter of the sigmoid colon, giving a rectosigmoid ratio less than 1. Another finding that may be seen on enema examination is a saw-toothed irregularity of the aganglionic portion of the colon secondary to abnormal peristaltic activity (Fig. C7.1).
Clinical signs of intestinal obstruction in neonates include failure to pass meconium, progressive abdominal distention, refusal to feed, and vomiting. An extensive differential diagnosis exists in cases of suspected neonatal intestinal obstruction and the combination of the clinical history, physical examination, and radiologic evaluation is required for eventual diagnosis.
Differential considerations of low intestinal obstruction include meconium plug syndrome, colonic atresia, meconium ileus, or anorectal malformations such as imperforate anus.
Meconium plug syndrome, also termed small left colon syndrome, is a common cause of distal neonatal bowel obstruction and thought to be related to functional immaturity of the ganglion cells. Findings on contrast enema include small caliber of the left colon extending to the splenic flexure with abrupt transition to dilated proximal colon (Fig. C7.2). Multiple filling defects representing meconium plugs may fill the left colon and may pass during the exam. Unlike Hirschsprung’s disease, the rectosigmoid ratio is usually greater than 1.
Meconium ileus occurs in patients with cystic fibrosis with thick meconium that obstructs the distal ileum. Water-soluble enema demonstrates microcolon and filling defects in the terminal ileum representing meconium plugs.
Ileal atresia results from congenital absence or complete occlusion of the ileal lumen thought to be the result of in utero vascular accident. Enema shows a microcolon and contrast may reflux into a normal terminal ileum with obstruction at the site of atresia.
CASE 8: HYPERTROPHIC PYLORIC STENOSIS
A 1-month-old male newborn presents with nonbilious projectile vomiting. Physical exam demonstrates an olive-shaped mass in the right upper quadrant.
Hypertrophic pyloric stenosis
Abdominal ultrasound and upper GI series may both be used to diagnose hypertrophic pyloric stenosis, though ultrasound is the examination of choice when hypertrophic pyloric stenosis is suspected. The benefit of ultrasound is that it may provide a rapid diagnosis without radiation exposure. Ultrasound examination also provides direct information on the anatomy of the pyloric canal, and unlike an upper GI series, there is no need for the patient to drink additional contrast material or to await gastric emptying.
Hypertrophic pyloric stenosis is a condition in which the antropyloric portion of the stomach becomes abnormally thickened and causes gastric outlet obstruction. The clinical presentation varies with the length of symptoms, but infants typically present with nonbilious projectile vomiting in the first 2 to 12 weeks of life secondary to functional obstruction. The vomiting may initially be intermittent, though the frequency will usually increase to follow all feedings and infants may develop weight loss and dehydration.
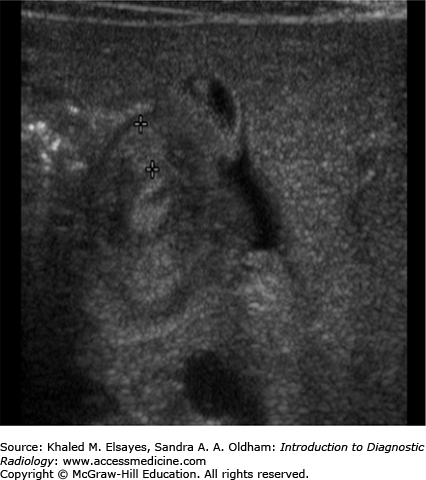
Ultrasound demonstrates variable distention of the stomach and decreased gastric emptying. On gray-scale ultrasound, the hypertrophied muscle is hypoechoic and there may be hypertrophy of the hyperechoic mucosa. Measurement criteria for the diagnosis of pyloric stenosis include muscle thickness greater than 3 mm and pyloric channel length greater than 15 mm (Figs. C8.1 and C8.2). During dynamic examination, the pylorus does not open and gastric hyperperistalsis without gastric emptying is identified.
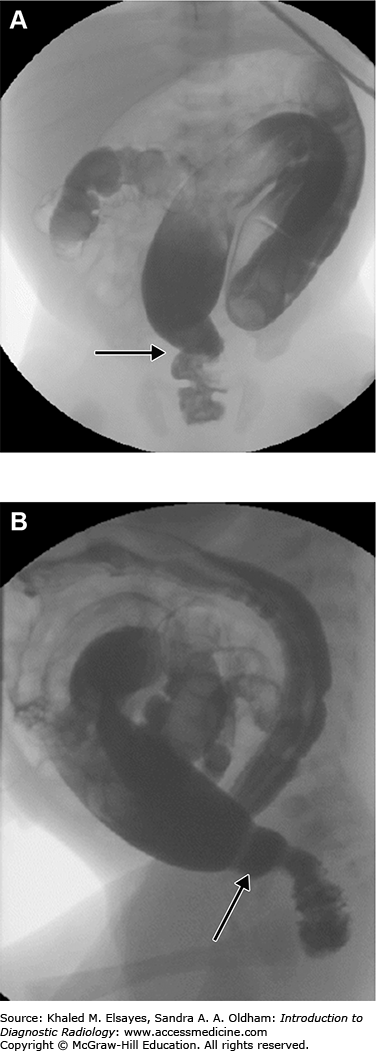
On upper GI examination, there is failure of relaxation of the prepyloric antrum, which is typically described as elongated pyloric canal. It may be seen as a string of contrast material through the antropyloric region, termed the string sign. Several linear tracts of contrast material within the canal separated by the intervening mucosa, named the tram track sign, may also be identified.
Other fluoroscopic observations include exaggerated gastric motility resembling a caterpillar, and another sign called the shoulder sign, where the hypertrophied pyloric muscle creates an extrinsic impression on the distal antrum. After the examination is performed and a diagnosis is made, excess barium should be removed from the stomach by nasogastric tube to prevent the risk of aspiration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree