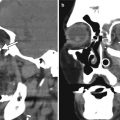
Fig. 4.1
Optic nerve and chiasm changes in chronic glaucoma. The patient has a history of long-standing normal tension glaucoma, left greater than right, treated with multiple topical medications. Axial (a) and coronal (b–d) T2-weighted MR images show diffuse optic nerve atrophy and hyperintensity as well as also atrophy of the optic chiasm, particularly on the left (arrows)
Numerous medical, laser, and surgical options are available for treating the various forms of glaucoma. Occasionally, B-scan ultrasound and ultrasound biomicroscopy can play a role in the diagnosis and follow-up of angle closure glaucoma, particularly when the mechanism of angle closure is called into question or gonioscopic findings are equivocal. Diagnostic imaging also serves a role in the post-therapeutic setting. B-mode ultrasound, CT, and MRI can depict certain glaucoma drainage implants, which may be encountered incidentally and should be appropriately recognized. In addition, these modalities can provide insight into the function of implants and are useful for the evaluation of certain complications, such as infection, hemorrhage, and device malposition, and to assess for the presence of underlying lesions that may be responsible for postoperative proptosis and ocular dysmotility. However, B-mode ultrasound, CT, and MRI have limited capacity for delineating the minute alterations of the fine anterior segment structures. When these structures must be assessed with adjunctive imaging, for which OCT and UBM are recommended. The particular indications for imaging and corresponding findings are described in further detail in the subsequent sections.
4.2 Pharmacological Management
Medical or pharmacological treatment is directed toward lowering intraocular pressure, which has been demonstrated to protect against further damage to the optic nerve head. Pharmaceuticals can decrease intraocular pressure by decreasing the production of aqueous humor by the ciliary body or by increasing the outflow through the trabecular meshwork or through the uveoscleral pathway. There are five major classes of drugs used for the long-term management of glaucoma: β-adrenergic antagonists, prostaglandin analogs, α-adrenergic agonists, carbonic anhydrase inhibitors, and cholinergic agonists. Conventional first-line treatment of glaucoma usually consists of a topical selective or nonselective β-blocker or a topical prostaglandin analog. Second-line drugs include α-agonists and topical carbonic anhydrase inhibitors. Parasympathomimetic agents, most commonly pilocarpine, are considered third-line treatment options. Hyperosmotic agents such as glycerol, isosorbide, and mannitol can be administered to lower intraocular pressure from very high levels in emergency situations, but not for long-term management. The effects of cholinergic inhibitors can be discerned on UBM or OCT, whereby successful therapy results in an observable increase in size of the aqueous outflow pathway, due to pupillary constriction. Topical prostaglandin analogs can also result in changes that are visible on diagnostic imaging, in the form of prostaglandin-associated periorbitopathy. This condition consists of atrophy of orbital and periorbital fat associated with enophthalmos due to inhibition of adipogenesis through FP receptor stimulation. Prostaglandin–associated perioribitopathy is fairly common with an incidence of 70 to over 90 %, depending upon the particular analog that is used. Orbit/maxillofacial CT or MRI can be used to depict the decreased orbital and periorbital fat volume without inflammatory changes (Fig. 4.2), which is particularly evident if the process is unilateral, and to exclude other conditions that may result in enophthalmos, such as silent sinus syndrome.


Fig. 4.2
Prostaglandin-associated periorbitopathy. The patient has a long history of topical prostaglandin use and displays upper lid ptosis and enophthalmos on exam. Sagittal CT image shows deepening of the upper eyelid sulcus, loss of the inferior orbital fat pads, and enophthalmos
4.3 Iridectomy and Laser Peripheral Iridotomy (LPI)
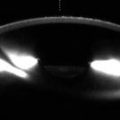
Surgical iridectomy and laser peripheral iridotomy (LPI) are procedures designed to minimize pupillary block, reduce peripheral iris-trabecular meshwork contact, and promote aqueous humor egress from the eye. Thus, these procedures are indicated for patients with acute-angle-closure glaucoma with pupillary block, although the LPI has largely supplanted surgical iridectomy but may be used in cases in which laser iridotomy fails. Alternatively, laser peripheral iridoplasty can be performed, which results in thinning of the peripheral iris, allowing for more aqueous humor exposure to contact the trabecular meshwork. Laser peripheral iridoplasty can be used for a crisis of acute angle closure and also in non-acute situations where there is residual angle closure despite a patent LPI. LPI can be performed using either Nd:YAG, argon laser, or both lasers in combination to create an opening that is usually 150–200 μm wide, which can be discerned by retroilluminating the iris using slit lamp biomicroscopy. In addition, the anterior chamber depth typically increases and the filtration angle widens following iridotomy, which can be measured on UBM or OCT (Fig. 4.3). UBM or OCT may also be obtained following LPI in order to evaluate residual angle closure. Several conditions can result in residual angle closure, such as plateau iris syndrome, pseudo-plateau iris syndrome, lens swelling, and failure to attain patency of an iridotomy. Plateau iris syndrome consists of angle closure due to anterior displacement of the peripheral iris despite an otherwise patent iridotomy. The ciliary body is rotated forward producing obliteration of the ciliary sulcus, resulting in an S-like morphology of the iris (Fig. 4.4). Pseudo-plateau iris syndrome is related to the presence of ciliary body or iris cysts which may not be readily apparent on slit lamp exam but can also produce angle closure. The formation of cystic and sheet-like epithelial invasion after surgical penetration of the anterior segment is rare. These lesions are best delineated on OCT or UBM (Fig. 4.5).




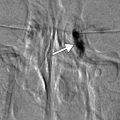
Fig. 4.3
Peripheral laser iridotomy. The patient incurred blunt trauma to the eye. Anterior segment OCT (a) shows anterior dislocation of the crystalline lens, which resulted in pupillary block angle-closure glaucoma. Following peripheral laser iridotomy, OCT (b) shows a patent peripheral iridotomy (arrow) and the deeper anterior chamber

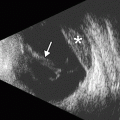
Fig. 4.4
Plateau iris syndrome. UBM shows anterior displacement of the lateral aspect of the iris at the site of iridotomy (arrow), where it blocks the canal of Schlemm

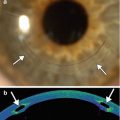
Fig. 4.5
Iris cyst. UBM shows an anechoic ovoid structure in the anterior chamber (arrow), obstructing the canal of Schlemm. An intraocular lens implant (bracket) is present and there is also retained intraocular lens cortical material (arrowhead) (Courtesy of Karen Capaccioli and Lois Hart)
4.4 Filtering Microsurgery (Trabeculectomy)
Trabeculectomy is often considered for patients who have failed laser surgery or pharmacotherapy. The procedure consists of creating a partial thickness scleral flap and removing an inner block of underlying corneal-scleral tissue, which typically includes trabecular tissue. In order to retain an opening (sclerostomy) from the anterior chamber to the subconjunctival space, a surgical iridectomy is typically performed to prevent peripheral iris prolapse into the sclerostomy. Subsequently, the scleral flap is sutured down over the sclerostomy at a desired tension to control the rate of outflow. Finally, the scleral wound is covered by conjunctiva and sutured tightly such that aqueous humor egress across the partial thickness sclerostomy is retained in the subconjunctival space creating a filtration bleb, which appears anechoic or hyporeflective on UBM and OCT, respectively. While tonometry provides an assessment of whether trabeculectomy is functioning, anterior segment imaging via OCT and UBM can provide a detailed assessment of the altered anterior ocular anatomy after trabeculectomy. In particular, the peripheral iridectomy and the sclerostomy site with fluid under the scleral flap can be delineated on UBM and OCT (Fig. 4.6). Bleb wall anatomy is better assessed by OCT, while in evaluating deeper structures, UBM is more effective. Due to its noncontact nature, OCT is particularly useful in the early postoperative stage. Furthermore, OCT can demonstrate features of bleb morphology that are not visible with the slit lamp examination. In particular, with respect to the appearance of blebs on imaging, two phenomena occur during the early post-trabeculectomy period in successful filtering operations: shading and stripping. With the shading phenomenon, there is poor visibility of sclera beneath the bleb. This decrease in visibility represents an increased diffuse water content commonly associated with successful filters. On the other hand, with the stripping phenomenon, there are multiple parallel hyporeflective layers or strips inside the Tenon’s capsule surrounded by hyperreflective areas representing fluid-filled channels with fine connective tissue septa. “Stripping” may indicate the presence of multiple drainage channels in successful filters.


Fig. 4.6
Trabeculectomy. Anterior segment OCT shows the peripheral iridectomy (bracket), the sclerostomy site with fluid under the scleral flap (arrowheads), and the elevated conjunctival filtering bleb
A major complication of trabeculectomy is scarring at the level of the conjunctiva–Tenon’s–episcleral interface, the scleral flap, the overlying episclera, or the internal ostium. This is often associated with reduction in size or absence of the bleb and effacement of the aqueous drainage pathway on imaging (Fig. 4.7). There are several options for minimizing the risk of scarring after trabeculectomy, including the use of mitomycin C (MMC) and collagen matrix implants. MMC is an antimetabolite used intraoperatively during trabeculectomy in order to prevent excessive postoperative scarring and thus reduce the risk of failure. Porous biodegradable collagen matrix implants, such as Ologen, may serve as an alternative to traditional antimetabolites such as mitomycin C and 5-fluorouracil in glaucoma filtration procedures. New blebs tend to contain microcysts that indicate the presence of aqueous flow channels, while older blebs tend to contain macrocysts with thin septations. In addition, older blebs associated with the use of antimetabolic agents tend to form avascular areas with thinning of the overlying conjunctiva (Fig. 4.8). The use of Ologen implants may result in fewer side effects. Ologen is designed to minimize scar formation by dissipating the fibroblasts, which randomly proliferate throughout the matrix pores. During the early postoperative period, the bleb is relatively prominent due to the space occupied by the collagen matrix implant, which can be discerned as a spongy appearing echogenic structure on UBM (Fig. 4.9).




Fig. 4.7
Failed bleb after trabeculectomy. OCT image shows that the iridectomy site is patent , but there is scarring of the conjunctiva and Tenon’s down to the sclera (arrowheads), and the bleb is flattened (arrow)

Fig. 4.8
Trabeculectomy with MMC and avascular macrocystic bleb. Anterior segment OCT (a) shows thinning of the conjunctiva overlying the bleb (arrow), which contains scattered thin hyperreflective septations. The corresponding clinical photograph (b) shows decreased vascularity in the conjunctiva overlying the bleb (Courtesy of Rebecca Tudor)

Fig. 4.9
Bleb revision with collagen matrix implant (Ologen). Anterior segment UBM (a) shows a patent surgical iridectomy (arrow) and an elevated filtrating bleb containing the collagen matrix (arrowheads). An intraocular lens implant is also present. Photograph of the Ologen Collagen Matrix (b) (Courtesy of Kestrel Ophthalmics)
A flat anterior chamber can result from overfiltration in the early postoperative period after trabeculectomy. This complication is usually attributable to decreased resistance to aqueous outflow through the sclera with resultant hypotony. It may be associated with the development of a choroidal effusion that may further decrease aqueous fluid formation. Left untreated, a flat anterior chamber may lead to secondary complications, including synechiae, cataract progression, and corneal endothelial decompensation. Detailed delineation of a shallow anterior chamber via OCT enables accurate measurements of the anterior chamber depth to be obtained, without applying pressure to the globe (Fig. 4.10). The normal depth of the anterior chamber is 3.13 ± 0.34 mm in women and 3.27 ± 0.28 mm in men.


Fig. 4.10
Shallow anterior chamber. UBM shows an anterior chamber with central depth of 0.79 mm. An intraocular lens implant is also present (Courtesy of Karen Capaccioli and Lois Hart)
Another rare complication of trabeculectomy is the formation of an overhanging cystic bleb, which lies on the surface of Bowman’s layer of the cornea and is covered by conjunctival epithelium. UBM or OCT often reveals multiple loculations with thin septa inside the bleb (Fig. 4.11). Indications for intervention include overfiltration leading to hypotony, foreign body sensation, lid retraction, lagophthalmos, and compromised visual acuity.


Fig. 4.11
Overhanging cystic bleb. The anterior segment OCT image shows a hypoechoic cystic bleb (arrows) that extends over the surface of the cornea (arrowheads)
4.5 Nonpenetrating Deep Sclerectomy
Nonpenetrating deep sclerectomy is a filtering surgery that is indicated to reduce the incidence of the postoperative complications encountered with the penetrating surgeries and is indicated for most of glaucoma (primary open-angle glaucoma, pseudoexfoliative glaucoma, pigmentary glaucoma, glaucoma associated with myopia, aphakic glaucoma, pseudophakic glaucoma, open-angle uveitic glaucoma, normal-tension glaucoma, and steroid-induced glaucoma), except angle closure and neovascular cases. The procedure consists of dissecting the conjunctiva and Tenon’s capsule and creating a limbus-based superficial scleral flap. A deeper scleral flap is dissected and the roof of Schlemm’s canal is removed. A space maintainer, such as a cylindrical collagen implant measuring 4 mm in length for a 0.5 mm diameter, is inserted, and the flap and conjunctiva are closed. Other space maintainer materials may include viscoelastics, HEMA (hydroxyethylmethacrylate), high reticulated hyaluronic acid, and polymethyl methacrylate (PMMA). Ultimately, the deep sclerectomy procedure creates a new outflow pathway for the drainage of the aqueous humor through the thin remaining trabeculo-Descemet’s membrane into the intrascleral reservoir without even entering the anterior chamber. The trabeculo-Descemet’s membrane prevents overfiltration and ensures a reproducible postoperative intraocular pressure. In addition, the presence of an intrascleral filtering space decreases the reliance on a subconjunctival filtering bleb. UBM and OCT are useful imaging modalities for evaluating the outflow mechanisms after nonpenetrating deep sclerectomy, since the bleb morphology correlates with intraocular pressure. In particular, successful surgery is indicated by the presence of high filtering blebs, a thin trabeculocorneal membrane, a hyporeflective suprachoroidal space, and blebs with low echogenicity based on UBM (Fig. 4.12) and thin bleb walls, large subconjunctival fluid spaces, and low bleb tissue reflectivity based on OCT. Furthermore, enhanced depth imaging spectral domain OCT can depict cupping reversal after nonpenetrating deep sclerectomy, which is mainly due to changes in prelaminar tissue thickness.


Fig. 4.12
Nonpenetrating deep sclerectomy. UBM shows the trabeculocorneal membrane (arrowhead) and the intrascleral lake of fluid (arrow) (Courtesy of Paul Harasymowycz MD)
4.6 Tube Shunt Surgery
Glaucoma drainage devices consist of an end plate, without or with a valve mechanism, and a tube (Fig. 4.13). The glaucoma (aqueous) drainage devices channel aqueous humor from the anterior chamber through a tube to an equatorial end plate in the posterior subconjunctival space, promoting bleb formation and tutoplast can be used to reinforce the tube and prevent erosion. Occasionally, the tube may be inserted into the posterior chamber sulcus in patients with pseudophakia, so as to reduce the likelihood of corneal endothelial loss and avoid the need for pars plana vitrectomy. Glaucoma drainage devices may be considered for glaucoma treatment that does not respond to pharmaceutical therapy or trabeculectomy. In addition, glaucoma drainage device insertion is commonly considered as the primary intervention for neovascular glaucoma, iridio-corneal syndrome, penetrating keratoplasty with glaucoma, and glaucoma following retinal detachment surgery. The drainage devices are most frequently positioned in the superotemporal quadrant of the orbit (Fig. 4.14). The inferotemporal quadrant (and occasionally the inferonasal quadrant) can also be used when the superior quadrant is not available for surgery (Fig. 4.15). However, the superonasal quadrant is generally avoided, due to the proximity to the trochlea.




Fig. 4.13
Intraoperative photograph of an Ahmed valve shows the end plate (arrow) and tube (arrowheads) components prior to implantation in the posterior subconjunctival space

Fig. 4.14
Superotemporal glaucoma drainage device. Coronal CT image shows the end plate with associated small bleb positioned along the superotemporal surface of the left globe (arrow)

Fig. 4.15
Inferotemporal glaucoma drainage device depicted on CT. The coronal CT image shows the hyperattenuating end plate of a silicone Ahmed valve along the inferolateral aspect of the left globe (arrow)
Several glaucoma drainage devices are commercially available, each with a unique design, biomaterials composition, and end-plate configuration. The Molteno and Baerveldt devices do not include valves. The single-plate Molteno has a surface area of 130 mm2, the double-plate Molteno implant has a surface area of 270 mm2, and the Baerveldt glaucoma implant (Advanced Medical Optics, Inc., Santa Ana, CA) has a large barium-impregnated silicone plate with surface areas of 250, 350, or 425 mm2; the Ahmed glaucoma valve has a surface area of 185 mm2 and the Krupin disk implant has a surface area of 184 mm2. These devices are made of different biomaterials: the Molteno and Ahmed devices are made of polypropylene whereas the Krupin and the Baerveldt are made of silicone. The end-plate biomaterial of the Ahmed valve was recently changed to silicone.
The barium-impregnated devices appear very radio-opaque, but should not be misconstrued as metallic foreign bodies on radiographs or CT (Figs. 4.16 and 4.17). On the other hand, the polypropylene devices are radiolucent, with similar attenuation as the orbital fat (Fig. 4.18). On MRI, the devices appear as low signal intensity on all sequences and are typically surrounded by a variable amount of fluid in the bleb (Figs. 4.19 and 4.20). However, the bleb should not exert significant mass effect upon the surrounding tissues. A mild degree of enhancement surrounding the bleb is typical. The blebs can be difficult to portray on B-mode ultrasound but appear as anechoic collections adjacent to the hyperechoic plate (Fig. 4.21). The tube portion of the glaucoma drainage device is best depicted on OCT or UBM (Figs. 4.22 and 4.23). The tube is typically implanted through the scleral limbus into the anterior chamber.





Fig. 4.16
Barium-impregnated glaucoma drainage device (Baerveldt shunt) depicted on radiography. The radiograph shows the radio-opaque implant in the superotemporal quadrant of the left orbit (arrow). The radiograph was obtained to screen for metallic foreign bodies prior to MRI

Fig. 4.17
Baerveldt shunt depicted on CT. Axial (a) and coronal (b) CT images show a thick, hyperattenuating superotemporal device (arrows)

Fig. 4.18
Radiolucent Ahmed valve with bleb depicted on CT. Axial (a) and coronal (b) CT images show a fluid-filled bleb (arrows) adjacent to a superotemporal radiolucent drainage device

Fig. 4.19
Baerveldt shunt depicted on MRI. Axial T2 (a) and post-contrast T1 (b) MR images show a small amount of fluid surrounding the hypointense plate (arrows) in the superotemporal quadrant of the orbit. There is expected enhancement of the bleb walls