Cell type
Major ultrastructural feature
Function
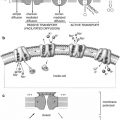
Chief cell
Slightly eosinophilic cytoplasm, few mitochondria
The active endocrine cell, producing the parathyroid hormone
Oxyphil cell
Rich eosinophilic cytoplasm, tightly packed mitochondria
May be able to produce parathyroid hormone
Transitional oxyphil cell
Less eosinophilic cytoplasm
Variant of oxyphil cell
Clear cell
Foamy and water-clear cytoplasm
Unknown, fundamentally inactive
Parathyroid hormone is a polypeptide that consists of 84 amino acids [13]. It has four principal actions: (a) to increase calcium absorption from the gastrointestinal tract; (b) to stimulate osteoclastic activity, resulting in resorption of calcium and phosphate from the bone; (c) to inhibit phosphate reabsorption by the proximal renal tubules; and (d) to enhance renal tubular calcium reabsorption. Parathyroid hormone secretion is controlled mainly by the extracellular calcium concentration. The parathyroid cell surface is thought to be equipped with a cation-sensitive receptor mechanism through which ambient calcium regulates the cytosolic calcium (Ca2+i) concentration and parathyroid hormone secretion. Activation of this receptor causes also activation of protein kinase C [3]. 1,25-Dihydroxycholecalciferol reduces the secretion of parathyroid hormone independent of any changes in calcium concentration. Parathyroid hormone is metabolized in Kupffer cells of the liver.
In patients with hyperparathyroidism, pathological parathyroid cells show defective sensing of ambient calcium. The cellular basis of this abnormality is unknown, although increased protein kinase C activity within abnormal parathyroid cells may be the mechanism. Pathological parathyroid glands also have an increased parenchymal cell content, although the extent of hypercalcemia appears more closely related to the defective secretory regulation than to increased parenchymal cell mass [5, 14].
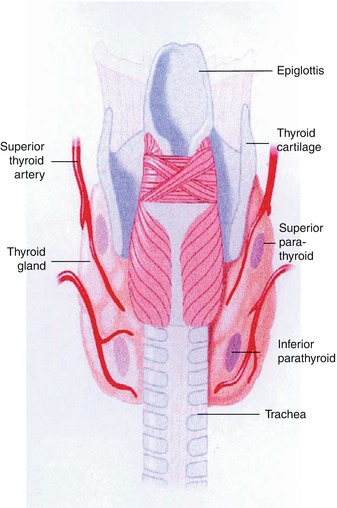

Fig. 8.1
A diagram showing typical locations of parathyroid glands
8.3 Hyperparathyroidism
Hyperparathyroidism has been diagnosed with increasing frequency in recent years due to awareness of the disease and to the laboratory advancement that allowed for routine chemistry screening. The condition is characterized by excess secretion of parathyroid hormone. The resulting biochemical changes, including increased levels of serum calcium and increased urinary excretion of calcium, may result in calcium wastage, nephrocalcinosis, urolithiasis, bone disease, and neuropsychiatric disturbances. Hyperparathyroidism may occur as a primary, secondary, or tertiary disease. It can also occur as eutopic and ectopic disease. In addition, it may have a familial origin, as in multiple endocrine neoplasia (MEN).
8.3.1 Primary Hyperparathyroidism
Primary hyperparathyroidism occurs due to neoplastic or hyperplastic parathyroid glands or when nonparathyroid tumors such as bronchogenic or renal cell carcinomas secrete ectopically parathyroid hormone or a biologically similar product. The incidence in the USA has been estimated at approximately 27.7 cases per 100,000 population per year [15]. The condition is more prevalent in females than males by a ratio of 3–1. More than 80 % of patients with primary hyperparathyroidism have a solitary adenoma. Hyperplasia – predominantly of chief cells – occurs in less than 20 % of patients. Parathyroid carcinoma is the cause in less than 1 % of patients, and very rarely, the condition is due to ectopic secretion of parathyroid hormone such as with renal cell carcinomas.
Primary hyperparathyroidism occurs as part of MEN. MEN is a hereditary syndrome that involves hyperfunctioning of two or more endocrine organs. Primary hyperparathyroidism, pancreatic endocrine tumors, and anterior pituitary gland neoplasms characterize type 1 MEN. MEN2A is defined by medullary thyroid carcinoma, pheochromocytoma (about 50 %), and hyperparathyroidism caused by parathyroid gland hyperplasia (about 20 %). MEN2B is defined by medullary thyroid tumor and pheochromocytoma. Both MEN1 and MEN2 are inherited autosomal dominant cancer syndromes. The gene responsible for MEN1 is a tumor suppressor gene located on chromosome 11.
Primary hyperparathyroidism is the most common manifestation of MEN1 (80 % occurrence) and is caused by hyperplasia of all four parathyroid glands. This is followed by pancreatic islet cell tumors and involvement of the pituitary gland [16–19]. There is a high frequency of carcinoid tumors of foregut origin with male predominance for thymic involvement and female predominance for bronchial lesions [17, 20]. Type1 MEN has a potentially lethal outcome with hemorrhagic peptic ulcer disease and metastatic pancreatic neoplasms [17]. Primary hyperparathyroidism is also associated with thyroid pathology in 15–70 % of patients [21, 22]. This includes thyroid carcinoma which has been reported in the range of 1.7–6.2 % (Table 8.2) of patients with primary hyperparathyroidism [21–30].
Table 8.2
Incidence of thyroid cancer among patients with primary hyperparathyroidism: cumulative literature data
Author | Year | # of patients | % with thyroid cancer |
|---|---|---|---|
Ogburn and Black [23] | 1956 | 230 | 4 (1.7 %) |
Nishiyama et al. [22] | 1979 | 420 | 13 (3 %) |
Prinz et al. [24] | 1982 | 351 | 16 (4.6 %) |
Hedman and Tisell [25] | 1984 | 426 | 25 (5.8 %) |
Attie and Vardhan [26] | 1992 | 948 | 31 (3.3 %) |
Burmeister et al. [27] | 1997 | 700 | 18 (2.6 %) |
Sidhu and Campbell [28] | 2000 | 65 | 4 (6.2 %) |
Bentrem et al. [29] | 2002 | 580 | 12 (2 %) |
Beus and Stack [21] | 2004 | 101 | 3 (3 %) |
Total | 3,821 | 126 (3.3 %) |
8.3.2 Secondary Hyperparathyroidism
Secondary hyperparathyroidism occurs when there is a condition causing chronic hypocalcemia such as chronic renal failure, malabsorption syndromes, dietary rickets, and ingestion of drugs such as phenytoin, phenobarbital, and laxatives, which decrease intestinal absorption of calcium. Secondary hyperparathyroidism is simply a compensatory hyperplasia in response to hypocalcemia. In this condition, reduced renal production of 1,25-dihydroxyvitamin D3 (active metabolite of vitamin D) leads to decreased intestinal absorption of calcium, resulting in hypocalcemia. Tubular failure to excrete phosphate results in hyperphosphatemia. Hypocalcemia along with hyperphosphatemia is compensated for by hyperplasia of the parathyroids to overproduce PTH [31].
8.3.3 Tertiary Hyperparathyroidism
Tertiary hyperparathyroidism describes the condition of patients who develop hypercalcemia following long-standing secondary hyperparathyroidism due to the development of autonomous parathyroid hyperplasia, which may not regress after correction of the underlying condition, as with renal transplantation.
8.3.4 Eutopic Parathyroid Disease
Parathyroid disease with typical location of glands (eutopic) represents 80–90 % of all cases [32]. There is a relatively fixed location for the superior parathyroids and they are found close to the dorsal aspect of the upper thyroid [11, 33]. On the other hand, inferior parathyroids have a more widespread distribution, which is closely related to the migration of the thymus. Inferior parathyroids are mostly located inferior, posterior, or lateral to the lower thyroid [11]. They may be very close to the thyroid and may be covered by or attached to the thyroid capsule and are sometimes adjacent to or surrounded by remnant thymic tissue. Interestingly, the parathyroid glands demonstrate a remarkably constant symmetry, which is helpful in the surgical exploration of eutopic disease [33].
8.3.5 Ectopic Parathyroid Disease
Superior parathyroid adenoma may have an abnormal supero-posterior mediastinal position, such as a retropharyngeal, retroesophageal, or paraesophageal site or the tracheoesophageal groove. The frequency of ectopia (up to 39 %) is similar for the right and left superior parathyroids [32]. Intrathyroid superior parathyroid adenomas are rare.
The more common ectopic inferior parathyroids are a well-established entity responsible for 10–13 % of all cases of hyperparathyroidism [32]. Ectopic tissue can occur from the angle of the mandible to the mediastinum according to the developmental and migratory aberrations. These sites include the mediastinum, thymus, aortopulmonary window, carotid bifurcation, and rarely thyroid, carotid sheath, vagus nerve, retroesophageal region, thyrothymic ligament, and pericardium [32, 34–36].
8.3.6 Parathyroid Adenoma
Parathyroid adenoma is a benign tumor that is usually solitary, although multiple adenomas have been reported in a low percentage. The tumor varies in weight from less than 100 mg to more than 100 g. The most commonly found adenomas, however, weigh 300 mg to1 g. The size was found to correlate to the degree of hypercalcemia [5].
Microscopically, the vast majority of typical adenomas are formed predominantly of chief cells, although a mixture of oxyphil cells and transitional oxyphil cells is also common. Adenomas formed of water-clear cells are very rare. A rim of parathyroid tissue is usually present outside the capsule of the adenoma and can serve to distinguish it from parathyroid carcinoma. The chief cells in adenomas are usually enlarged, and their nuclei are larger and more variable in size than in normal chief cells. Nuclear pleomorphism may be prominent; this is not considered a sign of malignancy but a criterion for discriminating adenoma from hyperplasia, which lacks this feature. The following variants of parathyroid adenoma may be recognized:
8.3.6.1 Solitary Adenoma
Solitary adenoma is found in 80–85 % of patients with primary hyperparathyroidism [33]. There is no significant predominance in location among the four parathyroids with each responsible for approximately 25 % of all solitary adenomas [32]. The remainder of parathyroid glands associated with single adenomas usually have lower weight and parenchymal cell mass than the average normal glands and show signs of secretory inactivity on electron microscopy [3].
8.3.6.2 Double or Multiple Adenomas
Double or multiple adenomas occur in up to 12 % of cases of primary hyperparathyroidism [37, 38]. Double adenomas are bilateral in 55–88% of cases and are seen predominantly in patients beyond the sixth decade of life [39]. These patients have more prominent symptoms and usually have higher parathyroid hormone and alkaline phosphatase levels than those with a solitary parathyroid adenoma or hyperplasia. However, symptoms and laboratory values do not enable the diagnosis of double adenoma. Preoperative detection of double or multiple adenomas with any imaging modality is not reliable [40]. Tc-99m sestamibi scintigraphy has a sensitivity of less than 37 % for detection of multisite disease [41, 42].
8.3.6.3 Cystic Adenoma
Cystic adenomas are thought to represent central necrosis or cystic degeneration of adenomas and account for less than 9 % of all parathyroid adenomas [43]. Contrary to the asymptomatic true parathyroid cysts which are due to embryological vestiges of the third and fourth pharyngeal pouches or enlargement of microcysts within the parathyroid as a manifestation of colloid retention [43, 44], cystic adenomas are frequently associated with hyperparathyroidism. Cystic adenoma may not be visualized on sestamibi studies.
8.3.6.4 Lipoadenoma
Parathyroid lipoadenoma, composed of hyperfunctioning parathyroid tissue and fatty stroma [45], is a rare entity that occurs in patients beyond the fourth decade of life [45]. Compared to typical adenoma, there is no gender predilection and no difference in terms of symptoms. On Tc-99m-sestamibi studies, the target-to-background signal ratio of lipoadenoma may be low due to the high adipose content of the tumor [45].
8.3.6.5 Oncocytic (Oxyphil) Adenoma
In contrast to the typical adenoma that is composed of chief cells or mixture of chief, oxyphil, or transitional oxyphil cells, oncocytic adenoma is formed of exclusively oxyphil cells or of more than 80 % of such cells. It is rare subtypes with an average size double that of chief cell adenoma. Because of the low rate of PTH production from oxyphil cells, oxyphil adenoma should be relatively large to overcome the inefficient hormone production and result in hyperparathyroidism [46]. It is found in the sixth or seventh decades and like the typical adenomas is more common in women [47]. Oxyphil adenoma has been reported to cause severe clinical and biochemical manifestations similar to that of parathyroid carcinoma.
8.3.7 Parathyroid Hyperplasia
Parathyroid hyperplasia affects the glands to varying degrees, and commonly one or two glands are of normal size even though microscopic signs of endocrine hyperfunction, described later, are present, at least focally, in all glands. Chief cell hyperplasia is the most common and is composed of chief cells or a mixture of chief cells and to a lesser extent oxyphil cells. The cells are arranged diffusely, in nodules, or there is a mixture of both patterns. Water-clear cell hyperplasia is rare and is characterized by substantial enlargement of most parathyroid glands. The large water-clear cells are usually arranged in a diffuse pattern [48].
In primary hyperparathyroidism, hyperplasia affects the glands asymmetrically. In secondary hyperparathyroidism, the hyperplastic glands are more uniformly enlarged than with primary chief cell hyperplasia, with two histological types (Table 8.3). In the tertiary form, the glands are more often markedly and asymmetrically enlarged with frequent prominent parenchymal cell nodules.
Table 8.3
Classification of parathyroid hyperplasia
Type | Major pathological features |
|---|---|
Primary hyperplasia | Uniform chief cells with some oxyphil and transitional oxyphil cells |
Secondary hyperplasia | |
Diffuse (classic) type | Cords, sheets, or follicular arrangement of cells replacing the stromal fat cells. Oxyphil cells are more frequent in this type. This type is indistinguishable from the primary type |
Adenomatous-nodular type | Cells are grouped in large islands or nodules. Necrosis is seen more frequently than in diffuse type |
Pathologically, it is difficult to differentiate primary chief cell hyperplasia of only one gland from adenoma. Both contain large numbers of active chief cells with cells characterized by aggregated arrays of rough endoplasmic reticulum and a large, complex Golgi apparatus with numerous vacuoles and vesicles. Secretory granules are frequently present in these cells. These changes indicate that most of these cells are in the more active phases of parathyroid hormone synthesis and secretion [49]. Molecular biology techniques used on pathological parathyroid tissue have shown that cell proliferation is monoclonal in many sporadic adenomas and in the largest glands of multiple endocrine neoplasia type I. This monoclonality has not been found in the smaller parathyroid glands of multiple endocrine neoplasia or in sporadic hyperplasia. Additionally, rearrangement of parathyroid hormone gene in chromosome 11 was observed in sporadic adenomas [50].
8.3.8 Parathyroid Carcinoma
Parathyroid carcinoma is a rare cause of hyperparathyroidism with a low incidence that does not warrant unique classification and management guidelines [51]. It can arise in any parathyroid gland, including ectopic and mediastinal, although the usual site of involvement is the normally located parathyroids. The tumor is found predominantly in patients between the ages of 30 and 60 years, with no sex preference, and is usually functioning. The tumors tend to be larger than adenomas and appear as lobulated, firm, and uncapsulated masses that often adhere to the surrounding soft tissue structures [52]. The involved glands usually weigh more than 1 g, and the diagnosis is restricted histologically to the lesions displaying infiltrative growth into the vessel or capsule, since pleomorphism can be seen in many adenomas. Patients with parathyroid carcinoma usually present with severe or atypical clinical picture. The PTH and calcium levels are usually significantly high. The bone and kidney are usually more frequently affected and with greater severity [46].
8.3.9 Hyperfunctioning Parathyroid Transplant
Autotransplantation of parathyroid tissue is performed in cases of recurrent, persistent type 1 MEN and symptomatic secondary hyperparathyroidism [19] in association with total parathyroidectomy. After total parathyroidectomy, the most normal glands, usually one or two, are used for the graft. They are diced into small fragments approximately 1–2× 1 × 1 mm with each fragment placed in an individual bed beneath the muscle sheath and between muscle fibers [19, 53]. The graft consists of a cluster of 10–25 parathyroid fragments. The remainder of the healthy gland (or glands) is cryopreserved for potential retransplantation [19, 40, 53]. Graft may be placed into the brachioradial muscle or flexor muscle group of the forearm or into the sternocleidomastoid muscle. A graft site in the forearm is preferred for accessibility for laboratory workup of parathyroid hormone levels and surgical re-exploration in cases of recurrent hyperparathyroidism [18]. The graft may be functional in 8–9 days after surgery [39].
After autotransplantation, recurrent hyperparathyroidism occurs in approximately 14 % of cases [54]. The hyperfunctioning transplant is a possible cause as is residual or ectopic diseased parathyroid tissue. A hyperfunctioning graft in the forearm is easily demonstrated with Doppler US or Tc-99m sestamibi scintigraphy [19, 55].
8.4 Consequences of Hyperparathyroidism
Excess secretion of parathyroid hormone promotes bone resorption and consequently leads to hypercalcemia and hypophosphatemia. The clinical presentation and complications of hyperparathyroidism depend on the rapidity of development and the degree of hypercalcemia. The following abnormalities may occur (Table 8.4):
Table 8.4
Consequences of hyperparathyroidism
Type of abnormality | Presentation |
|---|---|
Genitourinary | Nephrolithiasis |
Nephrocalcinosis | |
Renal insufficiency | |
Polyuria | |
Nocturia | |
Decreased urine concentrating ability | |
Gastrointestinal | Nausea |
Vomiting | |
Constipation | |
Increased thirst | |
Loss of appetite | |
Abdominal pain | |
Peptic ulcers | |
Heartburn (hypercalcemia causes increased gastric acidity) | |
Pancreatitis | |
Musculoskeletal: | Myopathy |
Muscle weakness | |
Osteoporosis | |
Osteomalacia | |
Bone and joint pains | |
Renal osteodystrophy | |
Pseudogout | |
Neuropsychiatric | Memory loss |
Anxiety | |
Sleeplessness | |
Confusion | |
Lassitude, coma | |
Depression | |
Impaired thinking | |
Psychosis | |
Others | Fatigue |
Hypertension | |
Pruritus | |
Metastatic calcification including cardiocalcinosis | |
Band keratopathy (present in the medial and lateral aspects of the cornea) |
Genitourinary such as nephrolithiasis and nephrocalcinosis.
Gastrointestinal including nausea, vomiting, constipation, peptic ulcers, heartburn (hypercalcemia causes increased gastric acidity), and pancreatitis.
Musculoskeletal abnormalities include myopathy, muscle weakness, osteoporosis, and others. In all forms of hyperparathyroidism, there is increased bone resorption associated with increased osteoblastic activity, leading to increased uptake of bone-seeking radiopharmaceuticals. See also Chap. 6.
Neuropsychiatric abnormalities as memory loss, anxiety, sleeplessness, confusion, lassitude, coma, depression, impaired thinking, and psychosis.
Others such as fatigue, hypertension, pruritus, metastatic calcification including cardiocalcinosis, and band keratopathy (present in the medial and lateral aspects of the cornea).
The five disease-specific symptoms are muscle weakness, polydipsia, dry skin and itching, memory loss, and anxiety. Overall, the symptoms, particularly the disease-specific ones, show significant decline after successful parathyroidectomy [56].
8.5 Management of Hyperparathyroidism
The routine blood chemistry screening has been behind the recent increase in the recognition of hyperparathyroidism. Surgery is the major and only current curative modality in treating primary hyperparathyroidism. It is recommended for all patients who are operative candidates and for many asymptomatic patients. Parathyroidectomy is successful in more than 90 % of cases in experienced hands, based on intraoperative localization by the surgeon [13].
Identifying the glands can be difficult, however, particularly with removal of multiple glands and with reoperation [57]. Three important factors contribute to successful surgical explorations: correct preoperative diagnosis, accurate preoperative localization of abnormal glands, and meticulous surgical technique [2]. Although the success rate is high in experienced hands, up to 25 % of the initial explorations fail because the abnormal glands cannot be located. Prolonged exploration was also found to result in a high incidence of recurrent laryngeal nerve damage [57]. Surgical re-exploration with violated anatomy is even more difficult and hazardous and can often be unrewarding. Preoperative localization of parathyroid lesions is thus desirable to reduce the incidence of missed lesions and to help avoid prolonged neck exploration. Since surgeons’ experience with neck exploration is dwindling due to the reduced incidence of thyroid surgery with the expanding use of iodine-131 for therapy of hyperthyroidism, preoperative localization of parathyroid lesions is even more important than before.
In recent years, minimal access parathyroid surgery (small incisions with gamma probe or endoscopic assistance) is increasingly becoming the operation of choice for single parathyroid adenomas [58]. Compared with bilateral neck exploration, it has a shorter hospital stay, less morbidity, and better cosmetic result [59]. The development of this minimally invasive surgical technique has placed an even greater emphasis on preoperative localization [60]. The forms that preoperative localization can take include computed tomography (CT), ultrasound, magnetic resonance imaging (MRI), arteriography, selective venous sampling, Tc-99m sestamibi (MIBI) scintigraphy, 18F-fluorodeoxyglucose positron emission tomography (FDG PET), and 11C-methionine PET.
8.6 Preoperative Localization
Surgical removal of the abnormal gland(s) remains the only cure for hyperparathyroidism. The classical surgical procedure is bilateral neck exploration with identifying and removing the abnormal gland(s). The success rate of this procedure is more than 90 % in experienced hands. Therefore, preoperative parathyroid localization was not an essential part of management except in cases of recurrent hyperparathyroidism or failure of the initial surgery. More recently, minimally invasive parathyroidectomy is becoming the procedure of choice for single parathyroid adenoma, which accounts for more than 80 % of cases of hyperparathyroidism. It has many advantages over the classical procedure including shorter hospital stay, less side effects, and better cosmetic results. This new surgical procedure has placed greater emphasis on the preoperative localization of abnormal gland(s).
Several imaging and non-imaging methods have been used to localize the abnormal glands and guide the surgeon. Invasive techniques include arteriography and selective venous sampling via neck vein catheterization. Although these techniques are reliable, they are expensive, time-consuming, and technically difficult and involve some risks. Noninvasive techniques are many, indicating that none of them is ideal. In general, older noninvasive techniques such as barium swallow, thermography, ultrasound, computerized tomography, and scintigraphy using selenomethionine-75 have not been considered very useful for preoperative localization. The morphologic imaging modalities, such as CT, ultrasound, and MRI, have the disadvantage that they cannot distinguish functional parathyroid tissue from other types of tissue. However, they provide excellent image resolution and contrast. Overall, their accuracy is inadequate and varies. Ultrasound, for example, is operator dependent and has a wide range of accuracy, with a range of sensitivity between 36 and 76 %. Computed tomography has a similar range, between 46 and 76 %. More recently, MRI has also been used with a reported sensitivity of 50–78 % [61–65].
8.6.1 Scintigraphic Imaging Localization
Parathyroid scintigraphy is not a screening study to be used in each patient with hypercalcemia of unknown etiology. It should be reserved for localization in patients with biochemically proven hyperparathyroidism.
Ideally, a radiotracer that is specific to the parathyroid glands alone should be used for localization. Unfortunately, such a tracer does not exist at the present time. Therefore, a number of tracers have been used in different methods with each one of them having its own limitations and advantages over the other ones.
8.6.1.1 Dual Isotope Method
This method is also known as the subtraction method and it was the first method to gain widespread acceptance for parathyroid imaging on the early 1980s. Multiple isotopes have been used to perform this method including thallium-201 (Tl- 201), Tc-99m pertechnetate, and iodine-123 (123I). Tl-201 accumulates in both the thyroid and parathyroid tissues. Therefore, thyroid activity should be subtracted from the image to allow for the identification of the parathyroid activity. This can be achieved by the use of another isotope that accumulates only in the thyroid tissue like Tc-99m pertechnetate or 123I. The thyroid image is then digitally subtracted from the thallium image. The resulting image is known as the subtraction image which represents activity within the abnormal parathyroid tissue.
There are many disadvantages of this method. The physical characteristics of Tl-201 are suboptimal resulting in a poor image quality. These include the photon energy of 69–80 KeV which is not ideal for the gamma camera. As well, the high radiation dose from this tracer limits the amount of dose administered. Digital subtraction of the images may be difficult due to technical problems like patient motion in between the two images. The reported sensitivity of this method for primary parathyroid adenoma varies from 42 to 96 % [66].
8.6.1.2 Dual-Phase Method
This method is based on the differential washout rate of sestamibi from the thyroid and the abnormal parathyroid glands. Taillefer and coworkers [67] introduced the concept of this single isotope, dual-phase method. It was observed that Tc-99m sestamibi washes out more rapidly from the thyroid than from the abnormal parathyroid tissue. It is assumed that the retention of the tracer in the abnormal parathyroid tissue is related to the presence of mitochondria-rich oxyphil cells.
This method is easy to perform as it requires a single injection of 370–740 MBq (10–20 mCi) of Tc-99m sestamibi. Early images are acquired at 10–20 min followed by delayed images at 2–3 h after tracer injection. These images can be acquired by parallel hole or pinhole collimators (Figs. 8.2, 8.3, 8.4, 8.5 and 8.6). Pinhole collimator is preferred as it improves the sensitivity for smaller lesions and provides the highest resolution among other collimators in addition to magnification of small structures [13]. Additional image of the chest should be also obtained as parathyroid adenoma can be found ectopically in the mediastinal area.
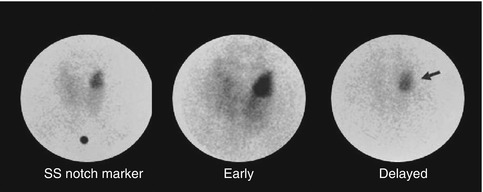
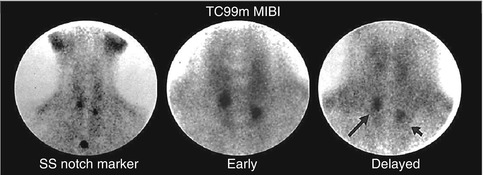

Fig. 8.2
Tc-99m Sestamibi study acquired 15 minutes and 90 minutes post injection using pinhole collimator. The delayed image shows differential clearance of activity from the thyroid gland with retained and intense uptake by a large parathyroid adenoma (arrow)

Fig. 8.3




Hyperplastic parathyroid glands (arrows) with persistent uptake on delayed Tc-99m sestamibi pinhole image
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree