LEARNING OBJECTIVES
1. Recognize and name four causes of a large unilateral pleural effusion on a chest radiograph or computed tomograph (CT).
2. Describe the difference in appearance of a pulmonary abscess and an empyema on chest radiograph or CT.
3. Recognize diffuse pleural thickening, as seen in fibrothorax, malignant mesothelioma, and pleural metastases.
4. Recognize a pneumothorax on an upright and supine chest radiograph.
5. Recognize imaging findings suggesting a tension pneumothorax or hydrothorax and describe the acute clinical implications.
6. Recognize a pleural-based mass with bone destruction or infiltration of the chest wall on a chest radiograph or CT; name four likely causes.
7. Recognize pleural calcification on a chest radiograph or CT and suggest the likely diagnosis of asbestos exposure (bilateral involvement) or old tuberculosis or trauma (unilateral involvement).
8. Recognize the typical chest radiographic appearances of pleural effusion, given differences in patient positioning, and describe the role of the lateral decubitus view to evaluate pleural effusion.
9. Recognize apparent unilateral elevation of the diaphragm on a chest radiograph and suggest a specific etiology with supportive history and associated chest radiograph findings.
The chest wall, pleura, and diaphragm enclose the outer lung. All are intimately associated with each other, which occasionally makes it difficult to determine the origin of a mass or disease process involving one or more of these compartments (Fig. 9.1). Disorders involving the chest wall, pleura, or diaphragm—resulting in a “pleural-based” mass—can arise from one of these compartments, an adjacent compartment, or another part of the body (as with metastatic neoplasms). Certain radiologic features can help determine the origin of an apparent pleural-based mass and narrow the list of diagnostic possibilities.
PLEURA
The pleura is composed of visceral and parietal serous membranes. The lungs and interlobar fissures are invested in the visceral pleura, whereas the parietal pleura lines the ribs, diaphragm, and mediastinum. The visceral and parietal pleura are continuous with one another as they are reflected around the hilum and the inferior pulmonary ligament. Inferiorly, the parietal pleura is situated within the costophrenic sulcus. The area between the two pleural layers forms a “potential space,” which can be enlarged when filled with fluid, cells, or air. Normally, there is approximately 1 to 5 mL of pleural fluid within this space (1). Because the total thickness of the pleural space and the normal visceral and parietal pleura is only 0.2 to 0.4 mm, the pleural layers are not usually identified on radiographs or computed tomographic (CT) scans of the chest except (a) when outlined by air or extrapleural fat, (b) where the visceral pleura invaginates into the lung to form the fissures, or (c) where the two lungs contact each other at junctional lines (2). The parietal pleura is separated from the ribs and intercostal muscles by a layer of fatty areolar connective tissue and a layer of endothoracic fascia. The parietal pleura receives vascular supply from and is drained by the systemic circulation, whereas the visceral pleura is supplied and drained mainly by the pulmonary circulation. Lymphatic drainage of the visceral pleura is by way of a lymphatic plexus that covers the surface of the lung just beneath the visceral pleura (3). These lymphatics do not connect with the pleural space. The parietal pleura is the primary drainage route for fluid in the pleural space, as it contains lymphatics that connect to the intercostal, internal mammary, and mediastinal lymph node chains (3). Except in rare circumstances (e.g., in some cases after cardiothoracic surgery or trauma), the right and left pleural spaces do not communicate with each other.

FIG. 9.1 • Association between the lung, pleura, and chest wall. The lungs are invested in the visceral pleura, whereas the parietal pleura lines the ribs and soft tissues of the chest wall, diaphragm, and mediastinum. The parietal pleura is separated from the ribs and intercostal muscles by fat and endothoracic fascia. The total thickness of the pleura and “potential” pleural space is only 0.2 to 0.4 mm.
A pleural-based density is one that, in some projection, presents a more or less sharp border indicative of a pleural surface, with a projected center lying outside the parenchyma of the lung. It may be in one of five locations: (i) extrapleural, (ii) parietal pleural, (iii) interpleural, (iv) visceral pleural, or (v) subpleural (Fig. 9.2). When associated with a rib lesion, an extrapleural lesion is most likely a hematoma (as from a rib fracture) or a tumor (with rib metastasis); when there is no rib lesion, it is likely a lipoma or lymphoma (when a patient is known to have lymphoma). Lesions involving the parietal pleura are often metastases, or if calcified, represent plaques from prior asbestos exposure. Interpleural density is common and can represent loculated pleural effusion or metastatic tumor. Visceral pleural lesions are uncommon but usually represent pleural thickening from asbestos exposure (whereas calcified asbestos-related plaques more frequently involve the parietal pleura) or metastases. Subpleural densities are parenchymal and generally do not have a sharp margin with the lung (Fig. 9.3), but on occasion tumors in the apex of the lung (so-called Pancoast tumors) can appear fairly well-circumscribed (Fig. 9.4). The etiology of many soft tissue masses, regardless of their origin, cannot be distinguished on radiography or CT of the chest, except in the case of lipoma, which has a characteristic low attenuation value on CT.

FIG. 9.2 • Radiologic pleural-based densities. A pleural-based density has margins that are partially or completely well-circumscribed, indicating contiguity with a pleural surface. There are five types of pleural-based densities, depending on the location of origin. An extrapleural density originates in the chest wall, and when it does not extend into the pleura and lung, it has a sharp medial margin where it contacts the parietal pleura. When the adjacent rib is involved, rib fracture with hematoma or neoplasm should be considered. Parietal and visceral pleural densities are usually asbestos-related pleural plaques, which may or may not be calcified. Interpleural densities most commonly represent pleural effusions; when intrafissural in location, they may be recognized on chest radiography by characteristic “beaking” at the ends of the fluid collection where the pleural layers of a fissure meet (producing a “pseudotumor”). Subpleural densities are parenchymal and usually have an indistinct lung parenchymal margin. If the lesion extends into the pleura and chest wall, all the margins of the lesion may be indistinct. Etiologies to consider when a subpleural density involves the pleura or chest wall are neoplasm and infection (especially fungal, mycobacterial, and actinomycotic).

FIG. 9.3 • Subpleural squamous cell lung cancer. A: Posteroanterior (PA) chest radiograph of a 67-year-old woman shows a mass in the left upper hemithorax (arrows) that is contiguous with the pleural surface. B: CT with lung windowing shows the mass abutting the lateral pleural surface and major fissure. C: CT with mediastinal windowing shows that the mass is contiguous with the pleural surface. Centrally, the mass contains areas of low attenuation, consistent with necrosis.
Pleural Effusions
Pleural effusions develop when there is excess pleural fluid produced, diminished resorption of fluid from the pleural space, or both. The fluid can originate from the pleura or be extrapleural in origin (Fig. 9.5). Pleural effusions are categorized as either transudates or exudates. The distinction is based on the specific gravity, protein, and lactate dehydrogenase (LDH) content of the fluid, with transudates having a specific gravity of 1.016 or less, a protein content of 3 g/dL or less, a ratio of pleural fluid protein to serum protein of less than 0.5, an LDH ratio (pleural fluid to serum) of less than 0.6, and an absolute pleural LDH level of less than 200 IU/L (4). Transudates develop primarily as a result of changes in microvascular pressure and plasma oncotic pressure, whereas exudates are caused by an altered pleural surface, with an increase in permeability or a decrease in lymph flow. The list of causes of pleural effusions is lengthy (Table 9.1), but more than 90% of effusions result from heart failure, ascites, pleuropulmonary infection, malignancy, or pulmonary embolism (5).
The appearance of an effusion depends on the patient’s position at the time of the radiologic examination and on the mobility of the effusion. In an upright person, fluid collects mainly in the lower pleural space, as long as it is freely mobile, creating a homogeneous opacity with a curvilinear upper border that is sharply marginated and concave to the lung. Fluid can collect in the fissures, creating a “pseudotumor” that conforms to the edges of the fissures and resolves with clearing of lung edema (Figs. 9.6 and 9.7). Occasionally, large quantities of pleural fluid accumulate in a “subpulmonic location” rather than in the general pleural cavity. In this case, the upper edge of the fluid mimics the contour of the diaphragm on the chest radiograph, creating the appearance of an “elevated diaphragm,” which usually peaks more laterally than normal. When this occurs on the left, the gastric air bubble and upper surface of the “hemidiaphragm” are separated more than usual. In supine patients, freely mobile fluid layers posteriorly, creating hazy, veil-like opacification of the affected hemithorax with preserved vascular shadows. Depending on the size of the effusion, this can be a subtle finding; when bilateral, it may not be detected at all, especially when small, or it may be confused with pulmonary edema. Other findings of pleural effusions in supine patients include blunting of the costophrenic angle (although this is often a false-positive finding) (6), capping of the lung apex, thickening of the minor fissure, and widening of the paraspinal soft tissues. Lateral decubitus views can be useful in verifying pleural effusions when the supine examination is equivocal, and they can allow determination of whether pleural fluid is mobile or not. The lateral decubitus view is much more sensitive than the upright view for the detection of pleural effusions; it can demonstrate as little as 5 mL of pleural fluid (Fig. 9.8) (7).

FIG. 9.4 • Pancoast tumor. PA (A) and lateral (B) chest radiographs of a 61-year-old man with right shoulder pain and a 40 pack-year history of cigarette smoking show a circumscribed mass (arrows) in the right apex. C: CT with bone windowing shows the mass filling the right lung apex and destruction of the right second rib (arrow).

FIG. 9.5 • Cerebrospinal fluid leak into pleural space. A: PA chest radiograph of a 42-year-old man who recently underwent partial corpectomy of the thoracic spine at several levels shows complete opacification of the right hemithorax and shift of the mediastinum to the left. B: Non–contrast-enhanced CT shows a large right pleural effusion, collapse of the right lung, mediastinal shift to the left, corpectomy changes, and continuity of fluid from the spine into the pleural space (arrow).
Table 9.1 COMMON CAUSES OF PLEURAL EFFUSION
Infection
Neoplasm
Cardiovascular disease (congestive heart failure)
Cirrhosis
Hypoproteinemia
Pancreatitis
Uremia
Subdiaphragmatic abscess
Trauma (hemothorax, chylothorax)
Occupational (asbestos)
Collagen vascular disease (systemic lupus erythematosus)
Hypothyroidism (often with pericardial effusion)
Pulmonary embolism
CT can detect smaller amounts of pleural fluid than can chest radiography (8). In addition, CT enables determination of whether fluid is loculated, the extent and localization of loculated fluid for purposes of drainage, assessment of pleural morphology (irregular thickening and focal masses suggest malignancy), evaluation of underlying parenchymal disease, and differentiation between pleural and parenchymal disease (aided by the use of intravenous contrast material). The attenuation value of pleural fluid on CT enables detection of a hemothorax (Fig. 9.9), which has a higher attenuation value than simple fluid; occasionally, a fluid–fluid or hematocrit level can be seen (see Fig. 8.26). A tuberculous empyema may appear as a low-attenuation pleural fluid collection on CT (Fig. 9.10). Clinical history guides the diagnosis when enhancing pleural masses result from prior talc pleurodesis (Fig. 9.11).
When a large unilateral pleural effusion is present, four causes should be considered: (i) infection (empyema); (ii) tumor (primary lung cancer, mesothelioma, metastases (Fig. 9.12), and lymphoma); (iii) chylothorax (secondary to tumor, most notably lymphoma, or ruptured thoracic duct); and (iv) hemorrhage (usually from trauma, whether iatrogenic or otherwise) (Table 9.2). Following drainage of a pneumothorax or pleural effusion, typically large and long-standing, the reexpanded lung may become acutely edematous. The edema usually develops within 2 hours of reexpansion, can progress for 1 or 2 days, and resolves within 5 to 7 days (Fig. 9.13).
Empyema is defined as pus in the pleural cavity. The diagnosis is made when the pleural fluid is obviously purulent, when organisms are identified in the fluid, or when the fluid has an elevated white blood cell count. An empyema is assumed to be present and drainage is indicated when there is associated pneumonia and the fluid pH is below 7.0 or the fluid glucose level is less than 40 mg/dL (9). The radiographic appearance of empyema is that of pleural fluid, which is usually unilateral but when bilateral is substantially greater in volume on the infected side (Fig. 9.14). In contrast to transudative pleural fluid collections, which typically have a smooth margin that is concave to the lung, an empyema will often have a smooth margin that is convex to the lung (Fig. 9.15). Certain CT findings are suggestive of empyema and other exudative effusions, including thickening and enhancement of the parietal and visceral pleura (creating the “split pleura sign”) after administration of intravenous contrast material (Figs. 9.16 and 9.17), thickening/inflammation of the extrapleural subcostal tissues, and increased attenuation of the extrapleural fat. Rupture of an empyema through the parietal pleura creating thick-walled intrathoracic and extrathoracic fluid collections is referred to as empyema necessitans, often from tuberculosis or invasive fungal infections (Fig. 9.18). In some cases, empyema can be difficult to distinguish from lung abscess. In general, there is a sharply defined border between an empyema and the lung with displacement and bowing of vessels and bronchi away from the empyema, whereas abscesses lack a discrete boundary between the lesion and the lung parenchyma. Empyemas are often elliptic and have a smooth inner surface, whereas abscesses are more often round and have a relatively thick wall. These features are not always reliable however, and occasionally it may be impossible to distinguish parenchymal from pleural fluid collections (10).
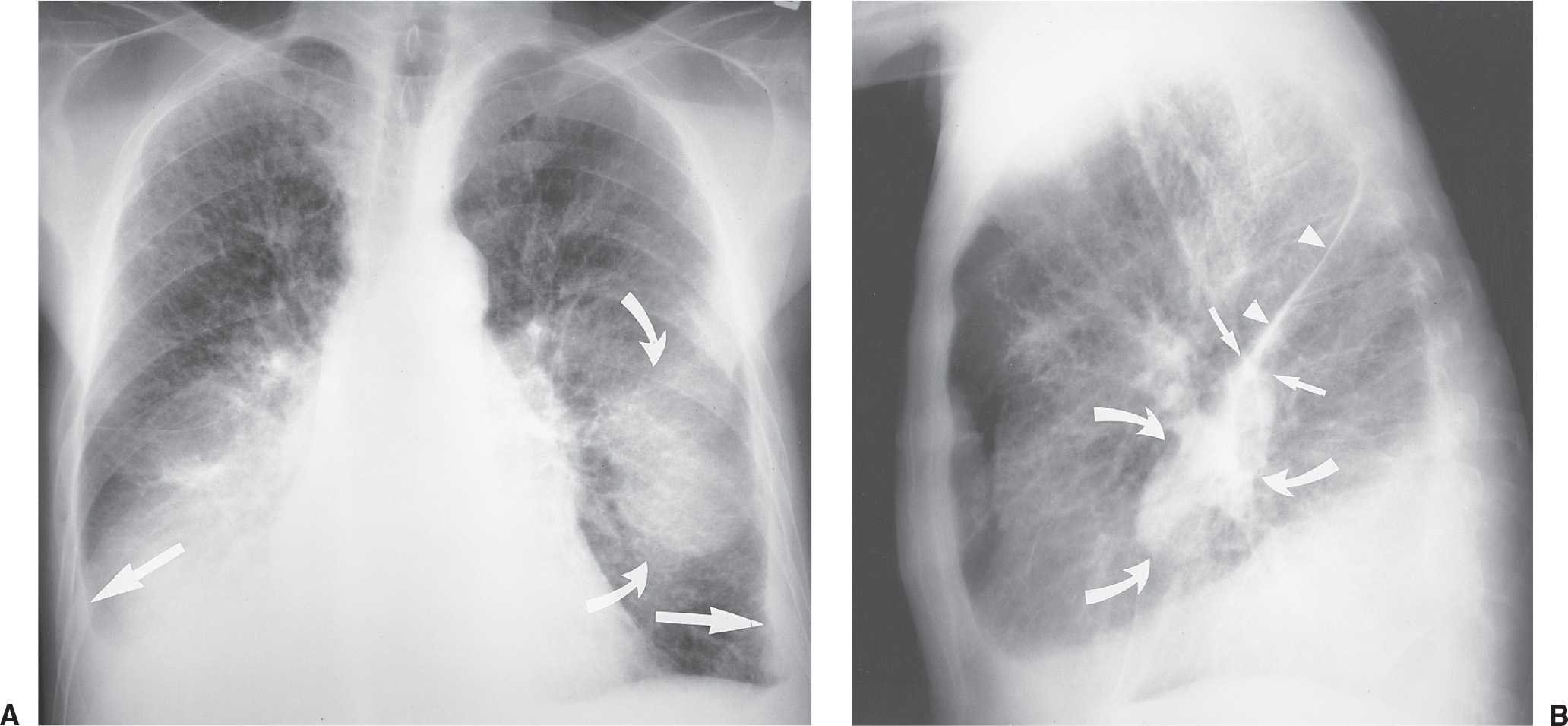
FIG. 9.6 • Pulmonary edema and pleural fluid pseudotumor. A: PA chest radiograph shows enlargement of the cardiac silhouette, interstitial pulmonary edema, and displacement of the inferolateral lungs from the chest wall and diaphragm by pleural effusion (straight arrows). There is a hazy “mass” in the left middle and lower hemithorax (curved arrows). B: Lateral chest radiograph shows that the “mass” or “pseudotumor” (curved arrows) blends in with the left major fissure (straight arrows); this is characteristic of pleural fluid within the fissure. The superior aspect of the left major fissure is thickened as a result of pleural fluid and subpleural edema (arrowheads).

FIG. 9.7 • Pleural fluid pseudotumor. A: PA chest radiograph shows a circumscribed ovoid mass in the right lower hemithorax (solid arrows) and thickening of the minor fissure (dashed arrow). B: Lateral view shows that the mass (arrows) is oriented in the direction of and superimposed on the major fissure. C: CT (bone windowing) shows that the mass is of fluid attenuation, representing pleural effusion (E), and is contiguous with the thickened major fissure (arrow).
A chylothorax contains fluid that is largely chyle (lymph of intestinal origin). Because chyle usually contains suspended fat in the form of chylomicrons, chylothorax fluid may be milky. Three main mechanisms account for chyle collections in the pleural space: (i) leakage from a discrete rupture of the thoracic duct or a large lymphatic vessel, (ii) a general oozing from pleural lymphatics, and (iii) passage of chylous ascites through the diaphragm (11). Approximately 50% of chylothoraces are of neoplastic origin, 25% are traumatic, and 15% are idiopathic (12). Lymphomas make up about 75% of the neoplastic lesions (13), and chylothorax can be the initial feature of lymphomas. The CT attenuation of chyle, despite its fat content, is usually indistinguishable from that of other effusions because chyle is protein rich (Fig. 9.19).

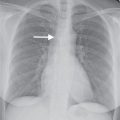
FIG. 9.8 • Positional appearances of pleural fluid on chest radiography and CT. A: PA upright chest radiograph shows apparent elevation of the right hemidiaphragm. The dome of the right hemidiaphragm appears to be displaced laterally (arrow), a clue to the diagnosis of pleural fluid collecting in a “subpulmonic” location. B: Anteroposterior (AP) supine chest radiograph of the same patient, 3 days later, shows hazy increased opacification of the right hemithorax secondary to pleural fluid layering posteriorly within the pleural space, now the most gravity-dependent portion of the pleural space. C: AP semi-upright chest radiograph of the same patient, 2 days after (B), shows a combination of pleural fluid layering posteriorly, which produces hazy opacity in the mid- and lower right hemithorax and dense opacity laterally (arrows). D: Right lateral decubitus chest radiograph of the same patient, taken on the same day as (A), shows pleural fluid freely layering against the now gravity-dependent lateral chest wall, from the costophrenic angle to the lung apex (arrows). E: CT of the same patient, performed on the same day as (B), shows a moderate- to large-sized right pleural fluid collection (E), with associated “passive” atelectasis of the right lower lobe (A).

FIG. 9.9 • Hemothorax. CT shows high-attenuation blood (H) in the right pleural space.
Hemothorax usually results from trauma, either blunt or penetrating trauma to the chest or iatrogenic trauma (such as with central venous catheter placement) (14). A rapidly accumulating pleural fluid collection following trauma is likely of arterial origin. High-pressure bleeding from systemic vessels may be rapid and persistent, with the formation of a tension hemothorax. CT of hemothorax may show areas of hyperdensity (15). With clotting of the blood, loculation occurs; if undrained, a hemothorax may eventually organize and cause pleural thickening (fibrothorax). An intrathoracic fluid collection is categorized as extrapleural if it causes inward displacement of extrapleural fat (Figs. 9.20 and 9.21). Extrapleural hematomas occur most commonly from high-energy blunt trauma and are often associated with rib fractures (Fig. 9.22) (16).

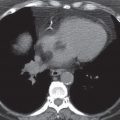
FIG. 9.10 • Tuberculous pleural effusion. CT scan shows a low-density left pleural fluid collection.

FIG. 9.11 • Talc pleurodesis. CT scans without (A) and with (B) intravenous contrast show dense pleural masses (arrows) related to prior talc pleurodesis. Biopsy of the masses showed hyalinized connective tissue with granulomas, associated with crystalline foreign-body material.

FIG. 9.12 • Malignant pleural effusion. PA chest radiograph of a 78-year-old woman with ovarian cancer shows a large right pleural effusion, leftward shift of the mediastinum, and complete collapse of the right lung.
Table 9.2 CAUSES OF A LARGE UNILATERAL PLEURAL EFFUSION
“ITCH”
Infection (empyema)
Tumor (primary lung cancer, metastasis, mesothelioma, lymphoma)
Chylous (ruptured thoracic duct, lymphoma)
Hemorrhage (iatrogenic or noniatrogenic trauma)

FIG. 9.13 • Reexpansion pulmonary edema. A: PA chest radiograph of a 78-year-old woman with metastatic breast cancer shows a large left pleural effusion, left lung atelectasis, and rightward shift of the mediastinum. These findings suggest tension hydrothorax. B: PA chest radiograph after placement of a chest tube and adequate drainage of pleural fluid shows reexpansion pulmonary edema on the left.
Malignant pleural effusions are usually the result of metastases (95% of cases) (17), with lung cancer accounting for 36% of cases (Fig. 9.23), breast cancer for 25% (Fig. 9.24), lymphoma for 10%, and ovarian and gastric carcinoma for 5% or fewer (Fig. 9.25) (18). Although pleural effusion is often the major component of metastatic disease to the pleura, other findings include pleural nodules (Fig. 9.26) or extensive pleural thickening similar to that of mesothelioma. When pleural metastases are extensive and unilateral, the CT findings may be indistinguishable from those of mesothelioma (19).
Malignant mesothelioma is a relatively rare primary tumor of the pleura. Approximately 80% of these lesions occur in individuals who have been exposed to asbestos (20). The lifetime risk for the development of mesothelioma in asbestos workers approaches 10%, and the average latency period is 35 years (21). Radiographic and CT findings include nodular or irregular thickening of the visceral and parietal pleura, variable ipsilateral volume loss in the hemithorax, ipsilateral pleural effusion, involvement of the interlobar fissures and mediastinal pleural surfaces, and often fixation of the mediastinum (Figs. 9.27 to 9.29) (22). Approximately 18% of cases are associated with invasion of the chest wall (Fig. 9.30) (22).
Pneumothorax
Pneumothorax is defined as a collection of air in the pleural cavity and is divided into spontaneous and traumatic types (Table 9.3). A pneumothorax occurring without an obvious precipitating traumatic event or in a healthy individual is a primary spontaneous pneumothorax. This type of pneumothorax is strongly associated with smoking and tall asthenic men (23). Most patients are between 20 and 40 years of age, and the male-to-female ratio is approximately 5:1 (24). The cause is nearly always the rupture of an apical pleural bleb (Fig. 9.31). Without treatment, the likelihood of another pneumothorax is about 40%, and the chance of recurrence rises with each episode (24).
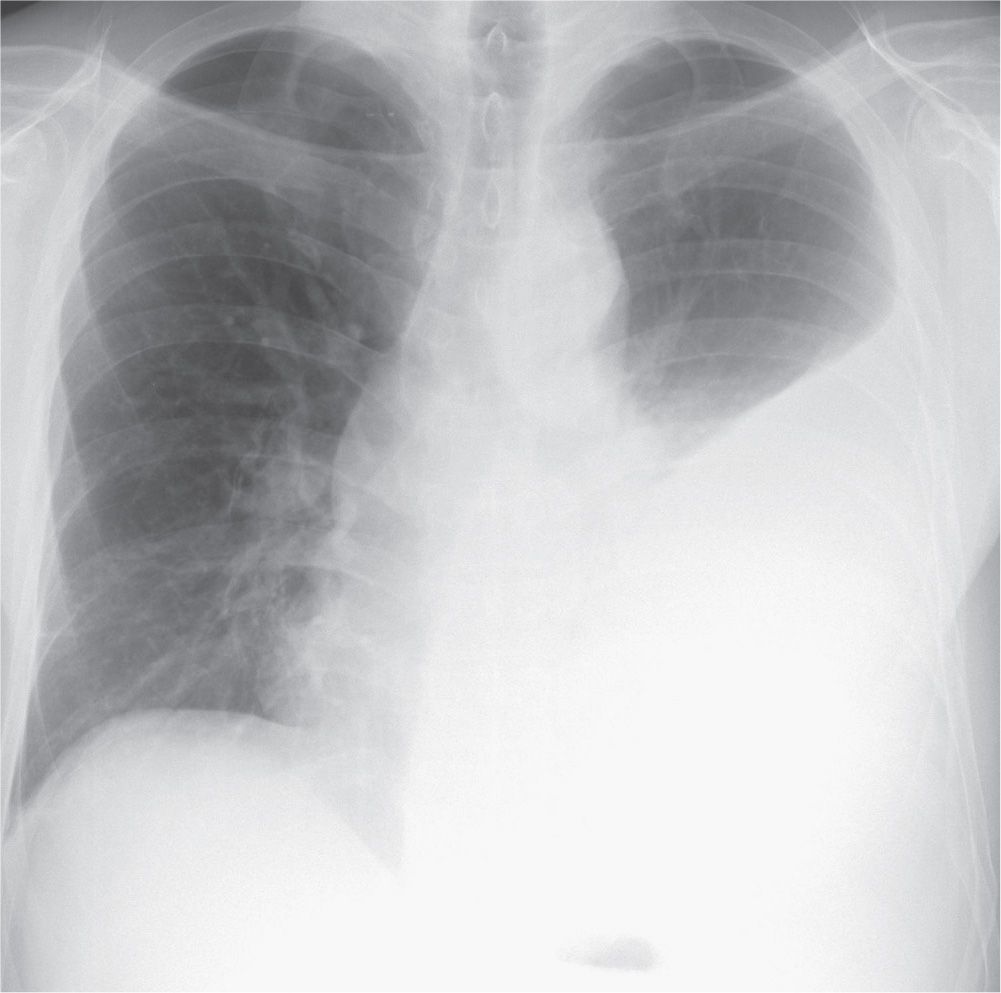
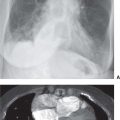
FIG. 9.14 • Tuberculous empyema. PA chest radiograph shows a large left pleural effusion. The differential diagnosis for a large unilateral pleural effusion includes empyema, hemothorax, malignancy, and chylothorax.

FIG. 9.15 • Empyema. A: PA chest radiograph of a 49-year-old woman with diabetes, left chest pain, and fever shows a large left pleural effusion with a medial margin that is convex toward the lung. B: CT scan shows a large pleural fluid collection with a lobulated contour and atelectasis of the left lung.

FIG. 9.16 • Empyema. A: PA chest radiograph of a 60-year-old man with right lower lobe pneumonia shows a large right hydropneumothorax with air–fluid level. B: CT shows a round collection of air and fluid in the right pleural space. The thickened and enhancing separated pleural layers create the “split pleura” sign. Air within an empyema can be secondary to thoracentesis, bronchopleural fistula, or, rarely, a gas-forming organism.

FIG. 9.17 • Empyema. A: PA chest radiograph of a 55-year-old man shows a large left pleural effusion and atelectasis of the left lung. B: CT shows an elongated ovoid collection of fluid in the left pleural space and collapse of the adjacent lung.
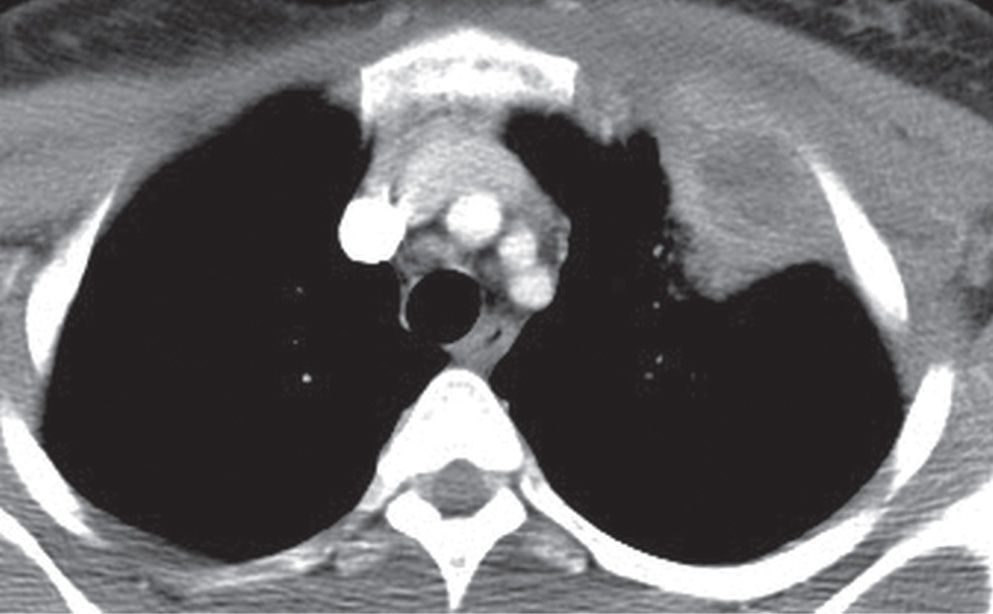
FIG. 9.18 • Empyema necessitans. CT shows a loculated, thick-walled left pleural fluid collection that has ruptured through the parietal pleura, creating both intrathoracic and extrathoracic components.

FIG. 9.19 • Chylous pleural effusion. CT shows a large left pleural fluid collection and rightward shift of the mediastinum.

FIG. 9.20 • Extrapleural hematoma. A: PA chest radiograph of a 45-year-old woman who fell and sustained multiple left rib fractures shows a left pleural-based mass that is convex toward the lung. B: CT shows a large extrapleural fluid collection that contains high-density material, a left rib fracture, and inward displacement of extrapleural fat (arrows).

FIG. 9.21 • Extrapleural hematoma. CT shows an ovoid extrapleural fluid collection associated with inward displacement of extrapleural fat (arrows) and right rib fractures.

FIG. 9.22 • Extrapleural hematoma. CT shows a right rib fracture, chest wall hematoma, and extrapleural fluid collection associated with inward displacement of extrapleural fat.

FIG. 9.23 • Malignant pleural effusion. CT of a 49-year-old man with AIDS and non–small-cell lung cancer shows a large left pleural effusion, enhancing pleural metastases (arrow), and rightward shift of the mediastinum.

FIG. 9.24 • Malignant pleural effusion. A: PA chest radiograph of an 83-year-old woman with metastatic right breast cancer shows a large right pleural effusion and interstitial lung disease. B: CT after drainage of right pleural fluid shows nodular thickening of the vascular structures and pulmonary septae on the right, characteristic of lymphangitic carcinomatosis.

FIG. 9.25 • Malignant pleural effusion. A: PA chest radiograph of a 57-year-old man with metastatic esophageal carcinoma who underwent esophagectomy and gastric pull-through shows a lobulated opacity in the left upper hemithorax. B: CT shows loculated pleural fluid (E) extending into the fissure. There is fluid in the intrathoracic stomach (arrow).
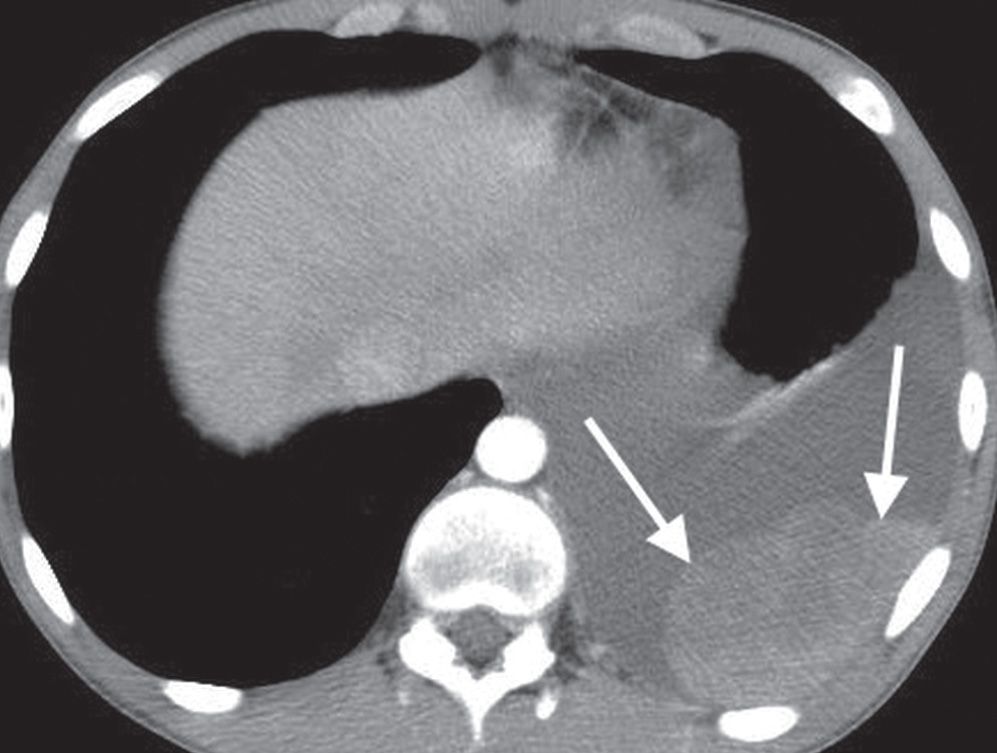
FIG. 9.26 • Malignant pleural effusion. CT scan of a 15-year-old boy with metastatic Ewing sarcoma shows a left pleura effusion and enhancing pleural metastases (arrows).
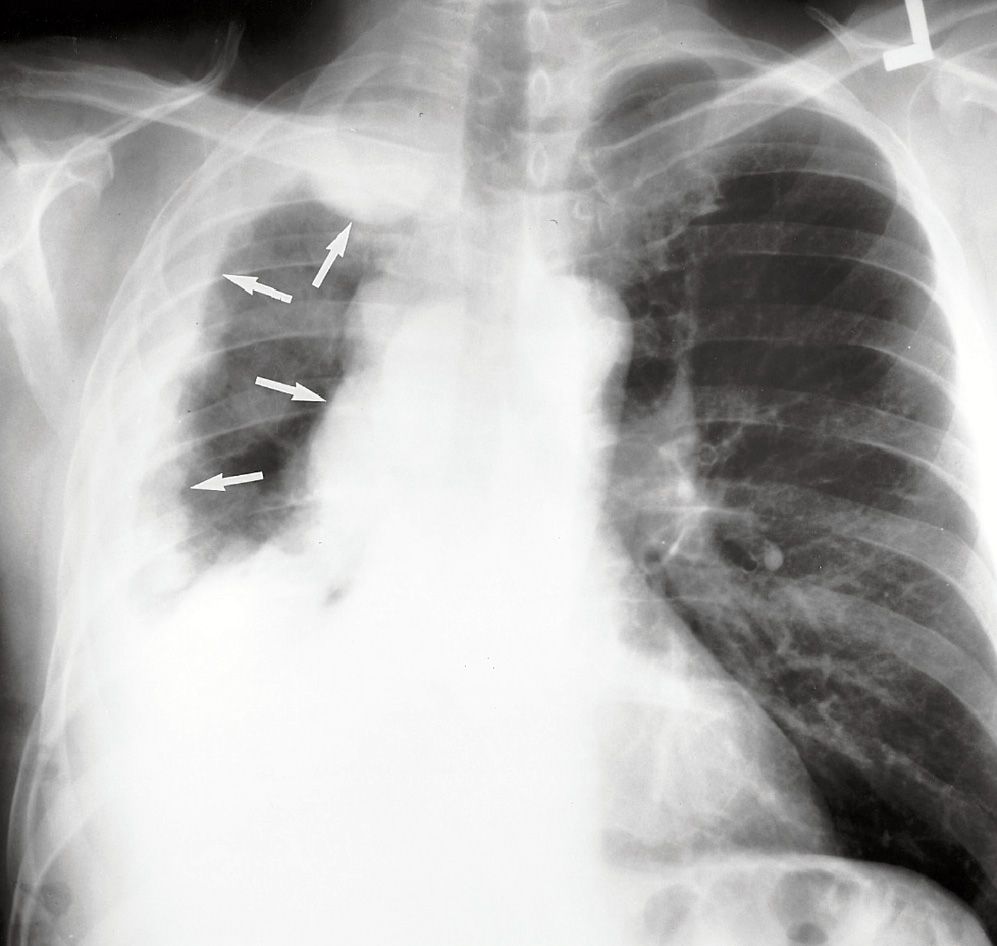
FIG. 9.27 • Malignant mesothelioma. PA chest radiograph of a 53-year-old man shows right pleural opacification with a lobulated contour that involves the entire pleural surface (arrows), characteristic of malignant mesothelioma. There is ipsilateral loss of lung volume.

FIG. 9.28 • Malignant mesothelioma. A: PA chest radiograph of a 43-year-old man shows an extensive right pleural process, extending into the minor fissure (arrows). B: CT shows that the pleural collection is of higher attenuation compared with simple fluid (consistent with tumor within the pleural space); wraps around the entire pleural surface, including the fissure (arrows); and has a lobulated contour.

FIG. 9.29 • Malignant mesothelioma. Coronal reformatted CT scan shows tumor involving the entire right pleural surface.

FIG. 9.30 • Malignant mesothelioma. CT with bone windowing shows right pleural thickening, lytic bone metastases (arrow), and calcified pleural plaques.
A pneumothorax developing without a precipitating traumatic event in a patient with predisposing lung disease is said to be a spontaneous secondary pneumothorax (Figs. 9.32 to 9.35). Chronic obstructive pulmonary disease is the most common cause of secondary spontaneous pneumothorax. About 0.5% of pneumothoraces are associated with lung metastases, of which 89% are caused by sarcomas, with osteogenic sarcoma being the most common (25,26). Catamenial pneumothorax is an uncommon disorder that occurs in women, probably caused by air entering the peritoneal cavity by way of the genital tract during menses and proceeding to the pleural cavity through diaphragmatic fenestrations. Catamenial pneumothorax occurs only in relation to the menses, appearing 1 day before or up to 3 days after menses. The pneumothorax is usually small and most often right-sided (87%) (27). Recurrence is a characteristic feature of catamenial pneumothorax, and it may be prevented by pregnancy or drugs that suppress ovulation.
Table 9.3 CAUSES OF PNEUMOTHORAX IN ADULTS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree