RADIOGRAPHIC CLASSIFICATION OF PNEUMOCONIOSES
The International Labour Office (ILO) has classified the plain radiographic abnormalities occurring in patients with dust exposure for the purpose of comparative epidemiological studies. This system provides a semiquantitative method for assessing the type and extent of abnormalities present. Classifications using this system are nonspecific, however, and it has little role in diagnosis. Furthermore, this system is complicated and difficult to remember unless used on a regular basis. However, limited familiarity with ILO system is appropriate (
Table 18-1).
A combination of letters and numbers is used to indicate the type and extent (profusion) of opacities present.
Opacity Type
The type of opacity, either small or large, is indicated by a letter, either small or large.
Small rounded opacities are well circumscribed and nodular. They are indicated as p, q, or r, depending on their diameter. Nodules up to 1.5 mm in diameter are “p,” opacities 1.5 to 3 mm are “q,” and those between 3 and 10 mm are “r.”
Small irregular opacities are linear or reticular in appearance and are indicated as s, t, or u according to their thickness. Opacities up to 1.5 mm in thickness are “s,” those 1.5 to 3 mm are “t,” and those 3 to 10 mm are “u.”
Large opacities are larger than 10 mm. These are classified as “A” if a single lesion or cluster of several opacities is more than 10 mm, but 5 cm or less in diameter. “B” indicates one or more opacities larger or more numerous than those defined in A, with a combined area not exceeding that of the right upper lung zone. “C” is used for opacities larger than B.
Profusion
The number of small rounded or irregular opacities is indicated by the term profusion. Profusion is graded using four numbers with the following definitions, most easily determined by comparison to a standard reference set of radiographs:
0: Small opacities absent or less profuse than indicated by 1
1: Small opacities definitely present but few in number (normal lung markings are still visible)
2: Numerous small opacities (normal lung markings partially obscured)
3: Very numerous small opacities (normal lung markings usually obscured)
These four profusion grades may be further subdivided. If in interpreting a radiograph, more than one of these four grades
was seriously considered but was determined not to be the profusion present, it is indicated following the final classification and separated from it by a slash. For example, if a patient is determined to have a profusion of 1, but 2 was also considered, this is indicated as 1/2. If no alternative was considered, the number is listed twice (e.g., 1/1). This leads to the following possibilities for profusion:
0/0 |
1/0 |
2/1 |
3/2 |
0/1 |
1/1 |
2/2 |
3/3 |
|
1/2 |
2/3 |
Also, 0/− may be used to indicate an obvious lack of small opacities, while 3/+ indicates a profusion much higher than 3/3.
Extent
In patients with large opacities, the lung is divided equally into three zones (upper, middle, and lower) using horizontal lines.
Accuracy of the ILO System
The National Institute of Occupational Safety and Health (NIOSH) has established a course and examination for the purpose of certifying physicians in the use of the ILO classification system. Completion of the course establishes the physician as an “A reader.” Passing an examination confers “B reader” status. B readers must be recertified every 4 years. Trained readers show less interobserver variability in interpreting pneumoconiosis radiographs than untrained physicians.
ILO categories are nonspecific and an abnormal classification need not indicate lung disease or the presence of pneumoconiosis. Approximately 5% of subjects without occupational exposure have radiographs interpreted as having a 1/0 profusion of small opacities, a classification usually considered to be abnormal and consistent with pneumoconiosis.
ASBESTOSIS AND ASBESTOS-RELATED DISEASE
Asbestos is a silicate mineral composed of various amounts of magnesium, iron, calcium, and sodium. Asbestos is found in nature in the form of thin fibers and is classified as amphibole or serpentine, depending on its fiber type.
The toxicity of asbestos appears to be related to the fiber form of the mineral and its durability after inhalation. Amphibole asbestos has fibers that are thin and straight, allowing them to penetrate deeply into the lung, and is most likely to result in disease. Amphiboles important in industry and often responsible for asbestos-related disease include crocidolite, amosite, and tremolite. Serpentine asbestos fibers are curved. Chrysotile is the only commonly used serpentine asbestos; it is less likely to result in disease than the amphiboles.
Asbestos has a variety of uses, particularly in the construction industry. Asbestos exposure may occur in the mining of asbestos, in the production of asbestos products and their installation during construction, and during repair or removal of asbestos-containing materials.
Asbestos-related abnormalities include asbestosis, asbestos-related rounded atelectasis, and asbestos-related pleural disease. A significantly increased incidence of mesothelioma, lung cancer, and extrathoracic neoplasms (e.g., peritoneal mesothelioma and gastrointestinal, renal, oropharyngeal, and laryngeal carcinomas) is also associated with asbestos exposure. Most patients with asbestos-related abnormalities have no symptoms.
Asbestosis
The lung disease associated with asbestos fiber inhalation is known as asbestosis (
Table 18-2). Asbestosis is defined as interstitial pulmonary fibrosis associated with the presence of intrapulmonary
asbestos bodies or asbestos fibers. Asbestos bodies are visible on microscopy and consist of translucent asbestos fibers coated with protein and iron. Amphiboles are usually responsible for asbestos bodies. Uncoated asbestos fibers are much more numerous in the lung than asbestos bodies.
Asbestosis results from inflammation associated with inhaled asbestos fibers. The development of asbestosis depends on the duration and intensity of exposure, although host-related factors such as tobacco smoking are
also involved. There is a significant correlation between the presence and severity of pleural disease and the presence and severity of asbestosis.
Symptoms of asbestosis include dyspnea and finger clubbing, usually occurring 20 to 30 years after the start of exposure. Symptoms are progressive even without continued exposure. Pulmonary function tests show restrictive abnormalities. Associated findings of airway obstruction may reflect associated smoking-related disease (i.e., emphysema) or asbestos-related bronchiolar fibrosis. Longstanding exposure may result in significant respiratory dysfunction leading to cor pulmonale and death. Risk of death is related to the severity of fibrosis determined clinically, functionally, or radiographically.
After inhalation, asbestos fibers are first deposited in the respiratory bronchioles and alveolar ducts, but deposition becomes diffuse with longer and more extensive exposure. In patients with asbestosis, the earliest changes of fibrosis are peribronchiolar. As fibrosis progresses, it involves alveolar walls throughout the lobule and interlobular septa. Honeycombing can be seen in advanced cases. Visceral pleural thickening often overlies areas of parenchymal fibrosis. In asbestosis, abnormalities are usually most severe in the lower lungs, in the posterior lungs, and in a subpleural location.
The diagnosis of asbestosis usually is based on indirect evidence, including a combination of chest radiographic abnormalities, restrictive abnormalities on pulmonary function tests, appropriate physical findings, and known exposure to asbestos. These findings are limited in accuracy, however, being both nonspecific and insensitive in early disease.
Many cases of asbestosis have resulted from exposure before, during, or in the years after World War II. The incidence of asbestosis is decreasing, and new cases with obvious findings are uncommon.
Radiographic Findings
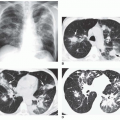
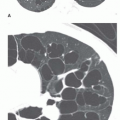
Asbestosis usually results in irregular reticular opacities (ILO classification s, t, or u) on chest radiographs. In its earliest stages, asbestosis appears as a fine reticulation in the lung bases, often best seen posteriorly on the lateral view (
Fig. 18-1A and B). As it progresses, reticulation becomes coarser and more obvious, partially obscuring the heart borders (known as the “shaggy-heart sign”). In more advanced stages, honeycombing may be visible and abnormalities become more extensive, involving the mid-lungs. Radiographic findings of asbestosis are not usually detected until 10 years after exposure, and the latent period is sometimes as long as 40 years. Also, as many as 10% to 15% of patients with proven asbestosis have normal chest films.
The combination of pulmonary abnormalies with typical pleural abnormalities should suggest the diagnosis (see
Fig. 18-1). However, plain film findings of pleural disease are absent in about 20% of patients with radiographic evidence of asbestosis.
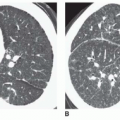
High-Resolution CT Findings
Asbestosis can result in a variety of High-resolution CT (HRCT) findings, depending on the severity of the disease. In general, HRCT findings reflect the presence of interstitial fibrosis and are similar to those seen in patients with usual interstitial pneumonia and idiopathic pulmonary fibrosis. Although no findings is specific for asbestosis, the presence of parietal pleural thickening in association with lung fibrosis is highly suggestive (see
Fig. 18-1C to E).
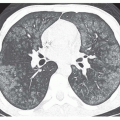
Thickening of interlobular septa, fine reticulation (intralobular interstitial thickening), traction bronchiectasis, architectural distortion, subpleural lines, and findings of honeycombing can all be seen, depending on the severity of disease. Honeycombing, common in advanced asbestosis, typically predominates in the peripheral and posterior lung bases (
Figs. 18-1,
18-2 and
18-3).
Patients with asbestosis usually show a number of HRCT findings indicative of lung fibrosis, and the abnormalities are usually bilateral and often somewhat symmetrical (see
Figs. 18-1,
18-2 and
18-3). The presence of focal or unilateral HRCT abnormalities should not be considered sufficient for making this diagnosis.
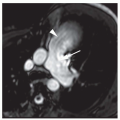
Parenchymal bands, linear opacities 2 to 5 cm in length, often extending to the pleural surface, are common in patients with asbestosis and asbestos exposure (
Fig. 18-4). These often reflect coarse scars or areas of atelectasis adjacent to pleural plaques or areas of visceral pleural thickening. Several parenchymal bands occurring in the same location may give the appearance of a
crow’s foot; this abnormality is often related to overlying visceral or parietal pleural thickening and may precede the development of rounded atelectasis. The presence of parenchymal bands does not necessarily indicate asbestosis.
Rounded Atelectasis and Focal Fibrotic Masses
The term
rounded atelectasis refers to the presence of focal lung collapse, with or without folding of the lung parenchyma (see
Table 18-2). It is typically associated with pleural disease and thus is common in asbestos exposure. Focal masslike lung opacities reflecting the presence of rounded atelectasis or focal subpleural fibrosis are seen in 10% of patients with significant asbestos exposure; they are usually related to adjacent visceral pleural fibrosis and measure 2 cm to more than 5 cm in diameter. It is important to distinguish these masses from lung cancer, which has an increased incidence in asbestos-exposed individuals.
Plain films show a focal mass in association with pleural thickening, often contacting the pleural surface. Rounded atelectasis may be associated with curving of pulmonary vessels or bronchi into the edge of the lesion, the so-called
comet-tail sign (see
Fig. 2-42 in
Chapter 2). CT is more accurate in making this diagnosis (see
Chapter 2).
Rounded atelectasis is most common in the posterior lower lobes and is sometimes bilateral or symmetrical. It may have acute or obtuse angles where it contacts the pleura.



 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access