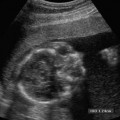
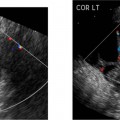
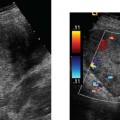
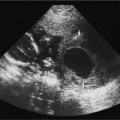
8 Tamoxifen Although this chapter is somewhat unique in the sense that the title does not describe a particular clinical symptom, the subject matter is extremely relevant and important because tamoxifen remains the most widely prescribed endocrine therapy for breast cancer. Dramatic findings showed that tamoxifen reduced the rate of breast cancer by an estimated 45% for healthy women over the age of 34 at increased risk. This was reported by news agencies worldwide in 1998 when the Breast Cancer Prevention Trial was halted early.1 Tamoxifen is a member of a drug class called selective estrogen receptor modulators (SERMs). These drugs appear chemically similar to estrogen and function by blocking the action of estrogen in breast tissue. Tamoxifen was the first SERM to be investigated extensively for its anti-cancer properties and it remains the standard of care for early breast cancer today. Breast cancer is the second leading cause of cancer deaths in women. It has long been a major public health problem for Americans, accounting for one of every three cancer diagnoses.2 Currently, this disease cannot be prevented, and most risk factors cannot be modified. The high rate of morbidity and mortality associated with breast cancer has sparked renewed research efforts directed at the treatment and prevention of this dreaded disease. Therapeutic regimes currently include surgery, radiation, hormones, and/or chemotherapy. Tamoxifen has been one of the most widely used, yet hotly debated treatments to date. Reported serious adverse effects, including an increased risk of endometrial cancer and venous thromboembolic events, initially raised the question of whether the net benefits outweigh the risks. There are now second-generation SERMs such as raloxifene. This drug was developed to avoid some of the undesirable estrogen agonist action of tamoxifen. Raloxifene is currently being used in the prevention and treatment of postmenopausal osteoporosis, with other potential indications still undergoing study. In the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, raloxifene compared with placebo increased bone minimal density by 2 to 3% and reduced the incidence of new vertebral fractures by 30 to 50%.3 Further studies have shown that raloxifene significantly decreases the incidence of breast cancer in selective populations; however, unlike Tamoxifen, any long-term use of raloxifene does not increase the risk of endometrial carcinoma.4 There is a new class of drugs challenging tamoxifen’s status as a treatment of choice for advanced breast cancer. Aromatase inhibitors (AIs) have recently gained attention as a possible alternative therapy that can be taken instead of or as additional therapy after the completion of long-term tamoxifen. In several large, international clinical trials, AIs, drugs that block the synthesis of estrogen from precursor hormones, were shown to be very beneficial in that they delayed breast cancer longer than tamoxifen in women with advanced disease, who are sensitive to estrogen and progesterone. AIs had fewer side effects than tamoxifen, such as vaginal bleeding and deep venous thrombosis; however, unfortunately, some AIs were associated with increased bone loss with resultant higher fracture rates and musculoskeletal pain.5 Tamoxifen is a synthetic, nonsteroidal drug that acts primarily as an estrogen antagonist on breast tissue and as an estrogen agonist on the endometrium. It was introduced in the early 1970s and was found to be widely effective as palliative therapy for women with advanced or recurrent breast cancer. Tamoxifen competes for estrogen receptors when administered to women who produce estrogen, thereby decreasing the net estrogenic effect. It has been hypothesized that tamoxifen deprives estrogen receptor–positive tumor cells of one of their necessary growth stimulatory factors.6 Another theory is that tamoxifen also interacts with estrogen receptors to induce synthesis of inhibitory substances that block the growth of mammary tumor cells and the cells of other organs as well.7–9 Tamoxifen also has a weak estrogen effect in hypoestrogenic women.10,11 About 50% of women with metastatic breast cancer benefit from tamoxifen.12 The beneficial effects are most apparent for postmenopausal women with estrogen receptor–positive tumors and therefore, tamoxifen and other SERMs are not indicated in women with estrogen receptor–negative tumors, who are generally treated with chemotherapy. By the 1980s, the success of tamoxifen in the management of advanced breast cancer provoked interest in its utility as adjuvant therapy in the treatment of surgically resected early-stage tumors. Overall survival statistics, as well as disease-free survival with reduced recurrence rates, have been documented for postmenopausal women when tamoxifen was added to other treatments. Several clinical trials also demonstrated prolonged disease-free survival for premenopausal women, as well as those with estrogen receptor–negative disease.12 The Scottish trial results suggested that postsurgical treatment with tamoxifen is more effective in prolonging survival than treatment at first signs of relapse. These data were used to support the rationale for preventative therapy in women at increased risk of developing breast cancer.13 Large, randomized, controlled clinical trials of adjuvant therapy for early-stage breast cancer demonstrated a 35 to 40% decrease in contralateral breast tumors for women treated with tamoxifen compared with controls.14,15 Additional reported potential benefits derived from the estrogenic action of tamoxifen included the stabilization of bone mineral loss, which may prevent morbidity due to osteoporosis, and lowered circulating cholesterol levels, which may significantly reduce the risk of death from coronary artery disease.16,17 Interestingly, tamoxifen is estrogenic on bones in postmenopausal women and anti-estrogenic on bones in premenopausal women. It is anti-estrogenic on certain parts of the brain, which can cause menopausal symptoms such as hot flashes, insomnia, and night sweats. Tamoxifen is estrogenic in the liver, producing an increase in blood clotting proteins, which slightly increases the risk of heart attacks and strokes, especially in those at high risk, such as cigarette smokers. Numerous studies report conflicting data on the effects of tamoxifen on total cholesterol.18 Experts generally agree that tamoxifen provides very effective treatment for women with all stages of breast cancer. Evidence now supports the use of long-term adjuvant tamoxifen therapy for extended periods of up to 5 years to prevent the reemergence of tumors.19 The benefits from 5 years of tamoxifen therapy, 20 mg daily, persists through 10 years of follow-up with no additional advantage to more prolonged treatment.20 Tamoxifen’s complex mode of action involves an estrogenic effect on the female genital tract. Estrogenic changes in the vaginal epithelium of postmenopausal women with breast cancer were first described in detail in the late 1970s.21,22 Laboratory studies then demonstrated tamoxifen-stimulated growth of human endometrial carcinoma transplanted into athymic mice and breast cancer cells in culture medium.23 Endometrial hyperplasia, polyps, uterine leiomyomas, uterine sarcomas, adenomyosis, and endometriosis were subsequently described in tamoxifen-treated patients by numerous researchers who implicated the prolonged estrogenic effects on the sensitive endometrium.14,24,25 The endometrial response to tamoxifen may even vary according to the menopausal status of the patient. A small study of 46 patients described polyps mainly in younger women treated with tamoxifen compared with predominantly hyperplastic and neoplastic lesions among postmenopausal women.26 The first case report of endometrial carcinoma associated with continuous postmenopausal tamoxifen exposure appeared in 1985 and many others soon followed.14,24–27 Most experts agree that proliferation of the endometrium with tamoxifen is associated with a significantly increased risk of the development of endometrial carcinoma. The relative risk of endometrial adenocarcinoma in patients with breast cancer has been reported as 1.72: 1.0 in women younger than 50 years, and almost 2.4 in women 70 years or older.28 In asymptomatic postmenopausal women, the estimated prevalence of endometrial cancer approaches 7 per 1000.29 In view of these statistics and the uncertain association between these two malignancies, a consideration is that a finite number of women will develop endometrial cancer, irrespective of their treatment protocol for breast cancer. Some researchers also raise the issue of whether the increase in incidence of endometrial carcinoma was a true phenomenon or perhaps due to an improved detection rate of very early tumors.7 A randomized Swedish trial of tamoxifen in 1846 postmenopausal patients undergoing primary surgery for early breast cancer with a median follow-up of 4 to 5 years reported a 6.5-fold higher occur-rence of endometrial cancer in the tamoxifen group compared with that of controls. In addition, the cumulative frequency of endometrial cancer was significantly greater in those who continued on tamoxifen compared with those who stopped treatment at 2 years.30 Supporting data by others have shown relative risks for developing endometrial cancer ranging from 1.7 to 4.6.31,32 Both the dose level and the duration of tamoxifen therapy are believed to be relevant to some degree. There may be a dose-dependency relationship associated with the proliferative effects of tamoxifen on endometrial tissue because the higher-dose Swedish trial had a more dramatic increase in endometrial cancer than other series using tamoxifen at lower doses of 20 mg daily.13,33 However, endometrial cancers have developed in patients on daily dosages ranging from 20 to 60 mg. A higher frequency of endometrial cancer with increasing duration of therapy has also been reported by other investigators.30 Endometrial cancer occurring after tamoxifen therapy is not of a different type with a worse prognosis than are such tumors in non–tamoxifen-treated patients.19 One study advocated pretreatment screening to identify patients at a higher risk of developing endometrial cancer. The authors reported a 17.4% incidence of asymptomatic endometrial lesions in 264 postmenopausal women who underwent pelvic sonography before starting tamoxifen at a daily dose of 20 mg. At 3 years of follow-up, the incidence of atypical endometrial lesions was significantly higher in women with initial lesions compared with those with a normal endometrium on pretreatment scans.34 There is currently no general consensus as to whether it would be beneficial to perform pretreatment pelvic sonography before tamoxifen therapy. Most researchers now concur that there is a definite increased risk of endometrial cancer, approximately two- to threefold, following chronic tamoxifen therapy for invasive breast cancer. However, this increased risk does not necessarily translate to a very high absolute risk, which can be affected by other factors such as hypertension, diabetes, obesity, and prior hormone therapy. Although endometrial cancer and rarer uterine sarcoma are the worse complications of long-term tamoxifen use, the majority of tamoxifen-induced uterine pathology is benign. Many abnormal uterine growths associated with tamoxifen are endometrial polyps. Figure 8–1 Normal postmenopausal uterus. Sagittal transvaginal sonography depicts the endometrium (cursors) as a 2 mm echogenic line in a patient on tamoxifen for 3 years, off therapy for 1 year, and then on Aromasin for the past year with numerous episodes of light spotting for 6 months. Figure 8–2 Mildly heterogeneous endometrium in retroverted uterus. Sagittal transvaginal sonography in a postmenopausal patient on tamoxifen for 5 years with elevated serum tumor markers, CA 125, and positive family history of ovarian carcinoma with 7 mm thick endometrium (cursors) containing tiny cysts (arrows). Although tamoxifen has proven valuable in the treatment of patients with various stages of breast cancer, some researchers object to its use as prophylaxis in healthy women until further information becomes available. A more stringent standard of safety is believed imperative for a primary prevention measure before it becomes acceptable for the general population.18 Others report that the benefits of tamoxifen in saved lives exceed the incidence of endometrial cancer, which is predominantly a low-grade, well-differentiated disease.35 However, the benefit:risk ratio must be assessed for each individual, especially when tamoxifen is being considered for prophylaxis. Regardless of how the drug is administered, informed consent should be obtained and patients should be counseled regarding its side effects. Gynecologic examination, PAP smears, endometrial biopsy, hysteroscopy, and pelvic ultrasound have been described as valuable methods of evaluating pelvic symptoms and complaints involving women on long-term tamoxifen therapy.24–26 The imaging approach to evaluating the female pelvis includes both transabdominal (TAS) and transvaginal (TVS) sonography. TAS uses probes up to 5 MHz with scans over a distended urinary bladder to provide a general overview of the entire pelvis. TVS became an established tool in the investigation of obstetric and gynecologic pathology because it affords high-resolution scanning capability with multifocal 7 MHz or higher vaginal probes used in close proximity to the pelvic organs with an empty bladder. The many technological improvements have included the introduction of vaginal transducers with color/power Doppler and three-dimensional (3-D) imaging capability. The vaginal technique is very well accepted by most premenopausal and postmenopausal women. TVS permits excellent visualization of the endometrium in the vast majority of patients and is far superior to TAS in this regard due to the improved resolution and near-field focusing. The endometrial–myometrial interface is much better depicted than was possible with full-bladder TAS (Fig. 8–1). The vaginal technique has been shown to consistently yield diagnostic information regarding subtle endometrial changes not detectable with transabdominal sonography.36–38 Meticulous scanning techniques may be necessary to image the endometrium of the retroverted or retroflexed uterus, which sometimes is at odd angles to the incident sound beam (Fig. 8–2). TVS can accurately measure endometrial thickness, which is the total measurement across the lumen of the endometrial cavity from one endometrial–myometrial interface to the other. This measurement should be performed with digital calipers in the sagittal plane at the site of maximal thickness, which is generally at or just below the uterine fundus. The value obtained actually represents two closely opposed endometrial layers. Endometrial fluid and the hypoechoic, inner compact layer of the myometrium should be excluded to avoid overestimation of endometrial thickness (Fig. 8–3). In experienced hands, sonographic measurements of the endometrium are usually in excellent agreement among observers and have been shown to correlate well with measurements from gross specimens.39,40 Figure 8–3 Endometrial fluid with tiny endometrial cysts. (A) Sagittal scan of the uterus in postmenopausal patient on tamoxifen for ~6 months demonstrates a large quantity of endometrial fluid (F) and numerous tiny cysts (arrows). (B) Coronal power Doppler scan demonstrates no areas of hypervascularity in this asymptomatic patient in whom the endometrial abnormalities were first noted incidentally on a renal sonogram.
Tamoxifen Effects and Mechanism of Action
Tamoxifen and the Endometrium
Ultrasound Imaging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree