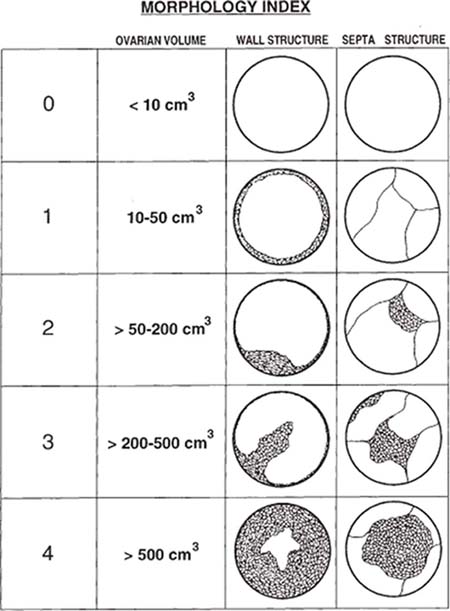
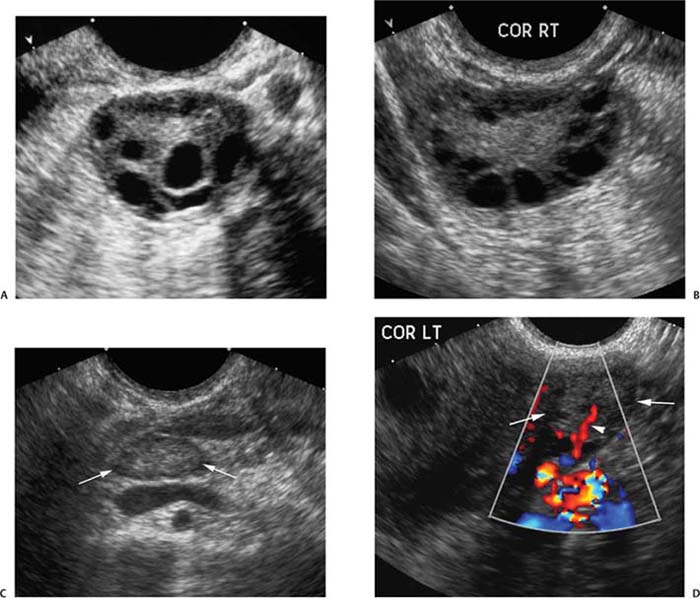
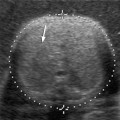
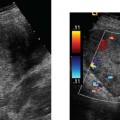
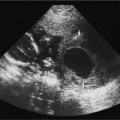
7 Family History of Ovarian Carcinoma The prevalence of ovarian carcinoma in the United States has been estimated at 30 to 50 cases per 100,000 women, translating into a lifetime incidence of one case per 70 women in the general population. With an anticipated 22,000 new cases and 16,000 deaths from the disease each year, ovarian cancer is the leading cause of death from gynecologic malignancies in this country.1 The preponderance (95%) of cases of ovarian carcinoma are sporadic in nature with no discernible pattern of inheritance. Some 5% occur in women considered at increased risk for the disease by virtue of first-degree relatives with ovarian cancer or a family history of one of three heritable syndromes discussed following here. Because ovarian cancer in its early stages produces few if any symptoms, and those symptoms that do result are typically nonspecific, ~70% of these tumors are detected at advanced stages (stages III and IV), when the process has already spread beyond the ovaries. Only ~20% are discovered while still in stage I. Because the 5-year survival rate for stage III or IV disease is 15%, whereas that for stage I exceeds 90%, the impetus for early detection is obvious.2–4 Certain general factors contribute to an increased risk for ovarian cancer: advancing age, nulliparity, North American or Northern European descent, family members with documented ovarian cancers, and a personal history of cancer of the colon, endometrium, or breast. Forty-five percent of ovarian masses removed from postmenopausal patients proved to be malignant as opposed to 13% from premenopausal women in one large study.5 In another report, 85% of all cases of ovarian cancer were found in women over 45 years of age.6 Conditions that seem to impart a measure of protection against ovarian cancer include more than one full-term pregnancy, oral contraceptive use, and breast feeding. The physiological factor common to all these is the interruption of ovulation for some period of time. A woman with one first- or second-degree relative with ovarian cancer faces a risk of developing the disease that is 3.1 times that of the general population (5% lifetime risk); the risk increases to 4.6-fold (7% lifetime risk) with two or three affected relatives7; some recommend annual screening to all women 25 years old and above with such a family history.8 A very small number of women (0.05% of the population) are known to be at markedly increased risk for the development of ovarian cancer by virtue of a family history of one of three hereditary syndromes. Patients with a family history of hereditary nonpolyposis colorectal cancer (Lynch II syndrome), breast–ovarian cancer syndrome, or site-specific ovarian cancer incur a lifetime risk of developing ovarian cancer approaching 40%. In this small group of high-risk women, in addition to annual screening, comes the recommendation for prophylactic oophorectomy at age 35 or whenever childbearing is complete.7–9 This is not applied to women who simply have a history of affected relatives without one of the defined syndromes. Malignant ovarian neoplasms are found with much higher frequency in women with one of three heritable syndromes than in the general population. They also occur at significantly younger ages in this small group. Women with hereditary nonpolyposis colorectal cancer syndrome develop ovarian cancer at a mean age of 40 years; with hereditary breast–ovarian cancer syndrome at a mean age of 52 years. This is in contrast to the general population in whom sporadic cases of ovarian carcinoma occur at a mean age of 59 years. Lynch II syndrome (hereditary nonpolyposis colorectal carcinoma syndrome) is an autosomal dominant trait with high penetrance. Affected women tend to develop colorectal cancers under the age of 45 years and have an increased risk for endometrial carcinoma. Women with this syndrome have an elevated risk of developing ovarian carcinoma.10 Histologically, these tumors are most likely to be cystadenocarcinomas. The recently identified BRCA 1 gene (on the long arm of chromosome 17) is the marker for the hereditary breast–ovarian cancer syndrome, which carries with it a 50% lifetime risk of developing ovarian cancer.11,12BRCA 2 is similarly implicated. The BRCA 1 gene is characterized as a tumor-suppressor gene; it is considered responsible for disease in 45% of families with multiple cases of breast carcinoma and the preponderance of the families with both breast and ovarian malignancies.13 The site-specific ovarian cancer syndrome is much less common than either the Lynch II syndrome or the breast–ovarian cancer syndrome. Only an increased incidence of ovarian neoplasm is seen without involvement of breast, colon, or other organs.12 The decision to implement a screening program is based on estimates of prevalence of the disease, cost per case discovered, altered clinical outcomes, and availability of a practical, sensitive screening test, among other factors. Despite its obvious morbidity and mortality, ovarian cancer has a relatively low incidence and prevalence (as compared, for example, with breast cancer, whose prevalence and incidence unquestionably make mammographic screening worthwhile). Current recommendations, therefore, are for screening only a small subset of the population considered at increased risk for the disease by virtue of a family history of ovarian cancer or the presence of one of the syndromes known to confer a substantially increased risk of ovarian malignancy. Screening of the general population is not advocated by any authorities at this time. Certain caveats with respect to screening bear mention. The detection of ovarian cancer by screening may still not be early enough in the evolution of the disease to affect ultimate outcome despite prompt intervention. The duration of preclinical disease has not been established; if it is short, screening at sufficiently close intervals to affect outcome may not be practically possible. There is also no assurance that tumors detected at an early stage by a screening program will exhibit the same biological behavior as those that are clinically manifested at the same stage.8 That said, there is still a perceived benefit to be derived from screening a selected population. Screening the high-risk patient is considered appropriate and advisable despite the current lack of definitive data to confirm improved outcomes from early detection. Logic dictates, however, that, because there is such a vast gap in 5-year survivals between early and advanced stages of the disease (90% vs. 15%, respectively), discovering and treating ovarian cancer in stage I will inevitably be of benefit. The goal in a screening program would understandably be the highest possible positive predictive value (PPV) for the process [i.e., (true-positive results/true-positive + false-negative) 100]. Clinical investigators have suggested that a PPV of less than 10% (i.e., nine negative surgeries for the discovery of each ovarian cancer) would not be acceptable to either clinician or patient.14 Screening programs have used the combination of CA 125 and transvaginal ultrasound. CA 125 is a protein in the blood that is increased in the majority of women with ovarian cancer.15 Screening using both CA 125 and transvaginal ultrasound has been studied in the general population16–20 and in women with an increased risk for ovarian cancer due to family history.21–23 Preliminary results suggest benefit of screening in the high-risk population with an overall specificity of 99.9% and a PPV of 26.8% in one study.24 The PPVs in the general population, however, are not high enough to justify large screening programs. A targeted family history is a critical element in the evaluation of a woman to identify if she is at increased risk for ovarian cancer. Patients with a pertinent history for familial or syndromic predisposition to ovarian malignancy should undergo a careful bimanual rectovaginal examination yearly.24 Unfortunately, the physical examination is relatively nonspecific and has limited sensitivity.25 Limitations include examiner experience, patient body habitus, and a variety of pelvic pathologies that can produce masses or masslike effects on the physical examination. Nonetheless, physical examination continues to be viewed, rightly so, as the proper starting point in the screening process. Serum CA 125, a tumor marker for ovarian cancer, does not have sufficiently high sensitivity and PPV to be used effectively for screening for ovarian malignancy. Although elevated in 85% of women diagnosed with ovarian cancer, it is also elevated in a variety of nonmalignant conditions, including pregnancy, liver failure, and endometriosis, and can be elevated from malignancies other than the ovary, such as breast cancer and colorectal cancer.15 Serum levels greater than 35 U/mL are generally considered abnormal.15,24,26,27 Overall, CA 125 is elevated in more than 90% of patients with epithelial cancers of the ovary of International Federation of Gynecology and Obstetrics (FIGO) stages II, III, and IV; unfortunately, fewer than 50% of women with stage I disease are found to have abnormal levels.28,29 This latter group is the very one in which early detection might have a profound effect upon outcome. Although other serum markers have been evaluated, none has been shown to have a sufficient PPV to be helpful in screening for ovarian cancer.30,31 Although the combination of CA 125 and transvaginal ultrasound has shown promise,25 the specificity of CA 125 levels is less useful in premenopausal women than in the postmenopausal group by virtue of a variety of physiological conditions (e.g., menstruation, pregnancy) and benign pathologies (e.g., endometriosis) associated with elevated CA 125 serum levels.14,24,43 The basis for ultrasound screening by itself for ovarian neoplasm was established before the availability of transvaginal scanning using transabdominal techniques with transducer frequencies in the range of 3.5 MHz. Although these early studies form the basis for the current approach, it is universally recognized that transvaginal ultrasound is the only acceptable method of evaluating the ovary for early-stage tumors.33–36 Three parameters are considered in the sonographic assessment of the ovary: gray-scale morphology, spectral Doppler waveforms and quantification, and color Doppler imaging. These three have been studied for specificity, sensitivity, and predictive values in screening for ovarian cancer; each will be discussed and its overall contribution to effective screening outlined. Essentially, all investigators attempting to define gray-scale characteristics of benign versus malignant ovarian neoplasms have established some form of morphological classification that assesses several parameters: size, wall thickness, number and thickness of septa, soft tissue excrescences (from walls or septa), and overall echogenicity.29,37–47 Several authors have devised morphological classification systems that take into account sonographic characteristics, including size, wall characteristics, number and thickness of internal septa, and presence of internal debris. They assign numerical values to each factor and derive a composite score that reflects the likelihood of malignancy. This allows for at least a semiquantitative assessment of the likelihood a mass is malignant, although, admittedly, there is some measure of subjectivity to the interpretation of the transvaginal ultrasound. Most of the classification systems have considerable common ground; we have chosen that published by DePriest et al47 by way of example (Fig. 7–1). Estimation of ovarian size is accomplished with the formula for the volume of a prolate ellipsoid (oval with flattened ends): volume = length × width × height × 0.5233 (or, by approximation, one half the product of the three dimensions). In premenopausal women, the normal ovary typically has a volume between 5 and 15 mL, decreasing in size with advancing age. In postmenopausal women, ovarian volume is usually less than 10 mL41 (Fig. 7–2). Although larger ovaries, those greater than 5 cm in largest diameter, have a higher risk of malignancy than smaller ovaries, the parameter of overall ovarian size is not as useful for identifying ovarian cancer as is the sonographic evaluation of ovarian parenchyma.38 The size of ovarian lesions does correlate somewhat with the risk of cancer because, in general, the larger the ovarian mass, the more likely it is to be malignant.37,40 The thickness and contours of the walls of an ovarian mass are particularly important for predicting malignancy.42,43 Solid tissue excrescences from the inner walls of a cystic mass and focal or diffuse thickening of the walls exceeding 3 mm are most worrisome for malignancy.38,40,44–47 Overall, the greater the wall thickness and the more the number of soft tissue projections present, the higher the risk of malignancy (Fig. 7–3, Fig. 7–4, Fig. 7–5). Figure 7–1 Morphology index for classifying ovarian tumors based on size, wall structure, and septa. (From Ueland FR, DePriest PD, Pavlik EJ, Kryscio RJ, van Nagell JR Jr. Preoperative differentiation of malignant from benign ovarian tumors: the efficacy of morphology indexing and Doppler flow sonography. Gynecol Oncol. 2003 Oct, 91: 47. Reprinted by permission.) Septa within an ovarian mass are evaluated with respect to number, thickness, and irregularity. Masses with many septa have a greater chance of malignancy than those with few. Those with thick septa, especially septa measuring more than 3 mm or septa with focal thickening, have an increased chance of malignancy. Although the number of septa does correlate somewhat with malignancy, it should be noted that mucinous ovarian tumors, whether benign or malignant, tend to have more septations than serous tumors. Thus, no specific number of septa nor any ratio of septations to tumor volume can be used to discriminate a benign ovarian cystic mass from a malignant one (Fig. 7–6, Fig. 7–7, Fig. 7–8).38,40,44,48 Figure 7–2
Heritable Syndromes that Correlate with Increased Risk of Ovarian Cancer
Screening for Women at Increased Risk of Ovarian Cancer
History and Physical Examination
Serum CA 125 Levels
Ultrasound
Gray-Scale Morphology
Size of Ovaries
Wall Features of Ovarian Mass
Septa within Ovarian Mass
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree