17 Pregnant Women with High Maternal Serum–Alpha-Fetoprotein Alpha-fetoprotein (AFP) screening was shown to be effective for detecting neural tube defects (NTDs) in the 1970s.1In 1991, the American College of Obstetrics and Gynecology (ACOG) endorsed offering maternal serum (MS)-AFP testing to all pregnant women. Since then, screening in the United States has become more widespread, and experience in this country and others has demonstrated considerable benefits from AFP screening, not only for the detection of NTDs, but also for several other fetal abnormalities (i.e., twins, ventral abdominal wall defects, and chromosomal abnormalities). In one study, either high or low MSAFP was associated with 34% of all major congenital defects.2 Further, even in the absence of multiple gestations and discrete fetal defects, earlier studies suggested that women with high MS-AFP had a much higher rate of adverse pregnancy outcomes.3,2 More recent publications indicate that this association holds true for fetal death and premature birth, but an elevated AFP may not be a risk factor for fetal growth restriction and preeclampsia.5–8 It is estimated that as many as 20 to 38% of women with unexplained high MS-AFP will suffer adverse pregnancy outcomes9,2; this information is another important benefit of MS-AFP screening. These fetal deaths occur mainly in the second trimester, and the risk appears to be directly related to the degree of MS-AFP elevation.4 California implemented a statewide screening program in 1986. It is now required by California state law that women who begin prenatal care before 20 weeks be offered MS-AFP screening. Currently, over 300,000 pregnant women in California are tested annually. Among the first 1.1 million women screened through the California AFP Screening Program, 1390 fetal anomalies (morphological and chromosomal) were detected (prevalence of 1.3/1000). These included 710 NTDs (417 anencephaly, 247 spina bifida, and 46 encephalocele), 286 ventral abdominal wall defects, 163 fetuses with Down syndrome, and 231 cases of other chromosomal anomalies. Impressively, of all anomalies detected in this program, nearly three quarters involved two organ systems: the neural axis (51%) and the ventral abdominal wall (21%). This distribution of “likely” fetal anomalies is especially germane to the sonologist examining women with elevated MS-AFP. These two groups of fetal defects (and many others) can now be accurately detected on targeted prenatal sonograms performed by experienced examiners. AFP is a glycoprotein produced initially by the yolk sac and fetal gut, and later predominantly by the fetal liver. At the end of the first trimester, it is present in the fetal serum in milligram quantities, and in the amniotic fluid in microgram quantities, and in the maternal serum in quantities measured in nanograms. In the fetus, serum AFP level increases until ~14 to 15 weeks and then falls progressively. In normal pregnancies, AFP from fetal serum enters the amniotic fluid through fetal urination, fetal gastrointestinal secretions, and transudation across fetal membranes (amnion and placenta) and immature epithelium. Detectable quantities of AFP in the MS gradually increase during gestation, peaking at 30 to 32 weeks and declining thereafter. MS levels are usually reported in multiples of the median (MoM) to standardize interpretation among laboratories. There are several potential ways that fetal AFP can enter the MS in abnormal quantities. Among fetal defects, the most common mechanism is through fetal cutaneous defects. These defects result in leakage of fetal serum proteins into the amniotic fluid, and secondarily into MS. Other abnormalities, including intrinsic placental abnormalities and maternal–fetal hemorrhage, also allow fetal AFP to mix with MS. In some cases, the precise mechanism for the fetomaternal transfer is not known (proximal gut obstruction, renal agenesis), and may be secondary to diminished fetal gut degradation or elevated fetal serum concentrations of AFP. It would be ideal if a single MS-AFP level could completely segregate normal from abnormal fetuses. Unfortunately, this is impossible owing to considerable overlap in MS-AFP levels between normal and abnormal pregnancies. Thus, the choice of a judicious cutoff value that maximizes detection of anomalies and minimizes the number of false-positive results is necessary for this screening program to be effective. Most screening programs in the United States have settled on a serum value of ≥ 2.5 MoM. Using this cutoff, ~90% of anencephalic fetuses, 75 to 80% of fetuses with an open spinal defect, 98% of fetuses with gastroschisis, and ~70% of fetuses with omphaloceles will be detected.11 Further, using 2.5 MoM as the cutoff has resulted in a reasonably low screen-positive rate (~4 to 5%). It is optimal to test MS between 16 and 18 weeks. Accurate dating is critical for AFP screening because serum AFP levels rise ~15% per week during the 16 to 18 week window. MS-AFP values are also corrected for maternal age, maternal weight, race, and the presence of diabetes (diabetes has a depressing effect on MS-AFP, so lower levels may be found in association with NTDs).12 In California, ~2% of screened women have elevated MS-AFP levels (≥ 2.5 MoM), and ~3% have MS-AFP levels ≤ 0.5 MoM. The latter group is discussed elsewhere. Roughly 6 to 15% of women with high MS-AFP have some type of major congenital defect, and this risk increases with the magnitude of MS-AFP elevation.2,13,14 If MS-AFP is elevated, then a nontargeted, standard antepartum obstetrical sonogram (level 1) is performed for the purpose of identifying easily recognized causes of “false-positives” (gestational age ≥ 2 weeks more advanced than estimated clinically, multiple gestations, fetal death, and obvious fetal defects). The intent of a standard antepartum obstetrical sonogram is to provide a general assessment of fetal/pregnancy health; it is performed according to the published guidelines endorsed by the American Institute of Ultrasound in Medicine (AIUM), ACOG, and American College of Radiology (ACR).15 The standard antepartum obstetrical sonogram is an important step in the triage of patients with high MS-AFP; impressively, approximately 20 to 50% of the elevated MS-AFP levels will be explained by findings on this preliminary sonogram (including the detection of a number of neural tube and abdominal wall defects).16,2 If the elevated MS-AFP is not explained by findings of the standard antepartum obstetrical sonogram, traditionally, the next step has been to counsel patients and offer amniocentesis for measurement of amniotic fluid (AF)-AFP. Among women who choose to undergo amniocentesis following an “unrevealing” sono-gram, > 90% will have normal AF-AFP (< 2.0 MoM), and no further diagnostic evaluation is done.18 If the AF-AFP is elevated (≥ 2.0 MoM), then acetylcholinesterase (an isoenzyme important in neurotransmission) is tested on the amniotic fluid sample. Acetylcholinesterase is present in association with exposed neural tissue (and occasionally with abdominal wall defects). High AF-AFP plus positive acetylcholinesterase is quite specific for a fetal defect. In most screening programs, karyotype testing is also routinely performed on the amniotic fluid specimen. If the AF-AFP is elevated (≥ 2.0 MoM), a targeted fetal sonogram (level 2) is offered. Among women with elevated AF-AFP, approximately one third of fetuses are anomalous.18 Similar to MS-AFP, the likelihood of a neural tube or other defect increases proportionately with the degree of AF-AFP elevation, but clearly not all of these fetuses will be abnormal. The targeted sonogram is performed to determine (1) whether any fetal anomaly is present (AFAFP may be false-positive); (2) if the fetus is abnormal, what the nature of the anomaly is (e.g., NTD versus omphalocele); and (3) if present, the severity of the anomaly and presence/absence of associated malformations (e.g., spinal level of myelomeningocele). AF-AFP testing is a highly sensitive method for detecting or excluding NTDs. The negative predictive value of a normal AF-AFP is ~97 to 99%, and elevated AF-AFP plus acetylcholinesterase allows > 99% accurate detection of NTDs.19,2 The specificity is 94.9%.20 High-resolution, targeted ultrasonography performed in conjunction with abnormal AF-AFP is also highly accurate in identifying anomalous fetuses (i.e., > 99% accurate).18,2 Nevertheless, there is a small, but important procedural fetal loss rate, 1/200 (0.5%), associated with amniocentesis. As a result, women with elevated MS-AFP have, in increasing numbers, opted to go directly from the serum AFP test to a targeted fetal sonogram, skipping the amniocentesis. The latter approach has become more popular in the last few years for two major reasons. First, sonographic detection of the “likely” anomalies associated with high MS-AFP has improved over the last 10 to 15 years. Expected rates of sonographic detection for neural tube and abdominal wall defects are currently > 90%.21–27 It is estimated that a complete, detailed, normal sonogram can now reduce the MSAFP-based risk of a neural tube or ventral abdominal wall defect by 95%.28,2 Second, going directly to a targeted sono-gram circumvents the small, but important procedural risk of fetal loss from amniocentesis. Indeed, the United Kingdom has adopted this paradigm and detailed, targeted sonograms are now routinely performed as the second diagnostic step in women with high MS-AFP. Some have cautioned against adopting a routine policy of circumventing the amniocentesis because (1) this approach will require a much larger number of targeted sonograms (i.e., 10 times as many), and the larger number of experienced examiners may not be available, or patients may be required to travel a long distance for the targeted sonogram; (2) even experienced examiners, especially as the prevalence of defects falls in the population scanned, may not detect as many defects as AF-AFP testing; and (3) skipping amniocentesis will cause potentially detectable chromosomal abnormalities to be missed.23,2 The last issue remains controversial, and multicenter consensus has not been reached. Some favor a paradigm in which targeted sonography follows a high MS-AFP, arguing that there is only a very small risk of an abnormal karyotype in a fetus without morphological defects. Recall that most of the unsuspected autosomal trisomies detected with AFP screening will occur in the low MS-AFP group, and that autosomal trisomies represent the minority of abnormal karyotypes found in women with high MS-AFP. These include mosaic trisomy 8 and trisomy 9.31,2 For example, trisomies 13, 18, and 21 account for only 28% of abnormal karyotypes in women with high MS-AFP, compared with 75% of abnormal karyotypes in women ≥ of age. Further, fetuses with autosomal trisomies 13, 18, 21 detected as a result of high MS-AFP often have sonographically detectable structural abnormalities.25,2 If the targeted sonographic fetal survey in a woman with elevated MS-AFP is normal, it has been estimated that the risk of a fetal chromosomal abnormality is only 0.6 to 1.1%, and sex chromosome aberrations (other than 45X) account for many (30 to 50%) of the chromosomal abnormalities in these fetuses.24–28 There is no right or wrong choice, all women facing the choice of targeted sonography versus amniocentesis should be fully informed of these controversies during their counseling. Decisions to perform an amniocentesis versus a targeted sonogram will vary according to patient (maternal age, other serologic markers, e.g., HCG and estriol, and personal choice) and institution (depending on availability of experienced sonologists). Although many patients will elect to have a targeted sonogram instead of amniocentesis, amniotic fluid testing should still be strongly considered in the following patients: (1) fetal position or maternal body habitus precludes an adequate sonographic fetal anatomical survey; (2) equivocal sonographic findings (e.g., abnormal posterior fossa, but spinal defect not seen); (3) experienced sonographic examiner not available; and (4) nonlethal anomaly detected on standard antepartum obstetrical sonogram for which karyotype testing is appropriate.33–35 Accurate sonographic diagnosis has become extremely important in light of AFP screening in pregnancy. If the preliminary, standard antepartum sonogram is unrevealing or an amniocentesis shows an elevated AF-AFP, a targeted fetal survey is performed. Because the most commonly encountered defects are those of the neural tube and ventral abdominal wall, the neural axis and ventral abdominal wall will be the most critical regions for scrutiny during the targeted sonogram. A focused examination of the neural axis in each fetus should include an assessment of overall cranial size and contour, ventricular size (transaxial diameter of ventricular atrium > 10 mm is abnormal), and posterior fossa, including cerebellar morphology and cisterna magna.36–39 At the University of California–San Francisco, we also include images of the cavum septum pellucidum as a check for forebrain malformations. The spine should be carefully examined in each fetus, including segment by segment images in the transaxial and sagittal planes from the craniocervical junction through the sacrum. Sagittal and transaxial images of the spine should demonstrate an intact dorsal skin line. The normal curvature of the spine should be documented, and the ossified posterior elements examined for abnormal splaying. The ventral abdominal wall of the fetus is examined, with focused attention on the umbilical cord insertion. The examiner should maintain a heightened sensitivity to the presence of bowel loops within the umbilical cord or floating in the amniotic fluid distant from the cord insertion/abdominal wall. Several other important fetal anomalies are associated with elevated AFP, and these potential defects should also be sought on the targeted sonogram (Table 17–1). Less common defects include fetal teratoma (pharyngeal, sacral), defects caused by the amniotic band syndrome (asymmetric cephaloceles, gastropleuralschisis), cystic hygroma, lesions that alter the placentomaternal barrier (e.g., placental chorioangioma, lakes, and abruption/hemorrhage), proximal fetal gut obstructions (e.g., esophageal and duodenal atresias), some renal abnormalities, and oligohydramnios.39 Thus, careful examination of the face, posterior neck, oropharynx, thorax, abdomen (including a normally filled stomach) should be performed.40 The limbs and digits should be assessed for abnormalities suggesting the amniotic band syndrome or the vertebral, anorectal, cardiac, tracheoesophageal fistula, renal, and limb (VACTERL) anomalies association. Amniotic fluid volume should be qualitatively or semiquantitatively assessed in addition to careful examination of the placenta. The most commonly encountered individual defects are discussed in the following section.
Maternal Serum–Alpha-Fetoprotein Screening
Sources of Maternal Serum–Alpha-Fetoprotein
How Patients Are Triaged in Maternal Serum Screening Programs
Ultrasound Evaluation
Increased Maternal Serum-Alpha-Fetoprotein: What Should You Look For?
Common A. Neural tube defects Anencephaly Myelomeningocele (Chiari II) Cephalocele B. Abdominal wall defects Omphalocele Gastroschisis Gastropleuralschisis associated with abdominal band syndrome or limb–body wall C. Multiple gestations Uncommon A. Cystic hygroma B. Renal abnormalities Finnish nephrosis (no defect observed sonographically) Pelviectasis C. Chorioangioma D. Teratoma (oropharyngeal or sacrococcygeal) E. Esophageal/duodenal atresia F. Placental subchorionic hematoma G. Maternal hepatoma |
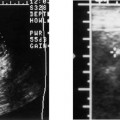
Anencephaly
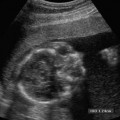
Anencephaly accounts for approximately half of all NTDs (Fig. 17–1). On average, anencephaly is associated with the highest AF-AFP and MS-AFP values of all NTDs, and ~90% will be detected by an MS-AFP ≥ 2.5 MoM. This is a lethal anomaly in which the bony calvarium is absent above the orbits. Normal cerebral cortex is absent. Some dysplastic “brain tissue,” representing angiomatous stroma, may be observed above the orbits, apparently floating freely in the amniotic fluid. Eighty-nine percent of fetuses with acrania had echogenic amniotic fluid on ultrasound performed between 11 and 13 weeks.41 Owing to its irregular shape and absence of recognizable normal morphology, it is unlikely to be confused for normal brain. One should be cautious, however, not to confuse an engaged fetal head (in which the convexity may not be well visualized) for anencephaly. This distinction is accomplished by the observation of amniotic fluid above the orbits and the calvarial defect. It is critical that this be diagnosed accurately because most patients will electively terminate their pregnancies following this diagnosis. Anencephaly can be diagnosed in virtually all affected fetuses after 14 weeks gestation.42
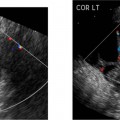
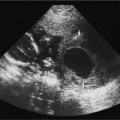
Myelomeningocele
Since the 1960s, the number of infants born with neural tube defects has been declining.43–45 The birth prevalence of myelomeningocele in 1970 was 1.3 per 1000 live births.43 This contrasts with a birth prevalence of 0.6 per 1000 live births after serum screening became available in the 1980s.43,2 This decrease is likely due to termination of pregnancies with a fetal neural tube defect. There has been a further decrease in birth prevalence to 0.41 per 1000 due to the Folic Acid Mandate of 1992 by the U.S. Public Health Service, which required fortification of foods with folic acid and encouraging daily folate supplements in women of child-bearing age.46
The myelomeningocele sac can be detected on sagittal or transverse views, but the sensitivity for detection of the spinal dysraphism is especially important if a sac is not seen or has ruptured. A myelomeningocele is suggested by a defect in the normal smooth dorsal skin line and splayed posterior ossification centers on the transaxial image (Fig. 17–2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree