The probability of nodal drainage to a specific ipsilateral lymph node level is directly related to the location and stage of the primary tumor. Table 8.2 is taken from Chao et al.2,3 and specifies the likelihood of pathologic lymph node involvement in both the clinically positive and negative neck by anatomic subsite. We will refer to this table frequently in the next section.
An excellent reference in the delineation of nodal levels as visualized on computed tomography (CT) slices has been published by the Radiation Therapy Oncology Group (RTOG) (http://www.rtog.org/site_map.html). Figure 8.1 demonstrates representative sections of these lymph node levels from the RTOG atlas.
Note that these atlases primarily refer to the untreated, node-negative neck. Recently, Gregoire et al.4 has published recommendations for the treatment of the node-positive or postoperative neck. Their recommendations can be summarized as follows:
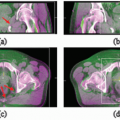
When lymph node level II is involved, the volume should be extended to include the retrostyloid space up to the base of skull (Fig. 8.2A).
When lymph node level IV or Vb is involved, the inferior border should be situated as such to include the supraclavicular fossa (Fig. 8.2B).
When a lymph node abuts a muscle or demonstrates clear extracapsular extension such as to involve the muscle, the entire muscle should be included in the CTV.
When a lymph node is located at the boundary of an uninvolved lymph node, the adjacent lymph node level should also be included in the CTV.
In a postoperative neck, the entire surgical field (“surgical bed”) should be included.
In general, a lymph node level is treated if the probability of metastasis in the absence of clinical involvement is >5%.
The risk of contralateral node involvement is also dependent on anatomic subsite, as well as the location of the tumor relative to midline and the T stage of the tumor. Anatomic subsites that thus tend to spread to the contralateral side most often are soft palate, base of tongue (BOT), hypopharynx, supraglottic larynx, and nasopharyngeal tumors.
It follows that the risk of bilateral nodal involvement is influenced by similar factors as contralateral disease. Table 8.3 depicts the risk of both contralateral and bilateral involvement according to primary site. Tumors of the nasopharynx, hypopharynx, BOT, and supraglottic larynx have the highest risk of bilateral involvement (nasopharynx is not listed in table).
Extracapsular extension (ECE) is a significant independent risk factor for local recurrence and distant metastasis, as
demonstrated by multiple studies. A study by Huang et al.5 examined the outcomes of 441 radical head and neck resections between 1982 and 1988 and found that in subgroup analysis, locoregional control was 31% with surgery alone and 66% with combined modality treatment in those patients with ECE. Interestingly, a recent study by Apisarnthanarax et al.6 showed that although lymph nodes >10 mm in diameter showed a higher probability of ECE at a given distance beyond the edge of the capsule, there was also a negative correlation between the frequency of ECE and the distance from the capsule.
When a patient is being treated postoperatively and has been found to have ECE, the CTV should be extended to the skin to account for this microscopic spread. At MSKCC, we use bolus in the case of ECE and treat the skin to full dose.
TABLE 8.1 Lymph Node Levels | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
TABLE 8.2 Incidence and Distribution of Lymph Nodes in N0 and N+ Neck | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
CTV1 – Highest dose region. Margin given to either the GTV (including involved lymph nodes), if definitive treatment, or gross residual disease, if postoperative treatment.
CTV2 – Intermediate-dose region. High-risk but clinically uninvolved regions. This includes the tumor bed if in the postoperative setting.
CTV3 – Low-dose region. Regions at a lower risk for microscopic disease.
If the lower neck contains involved or at-risk lymph nodes (such as adjacent involved lymph nodes) or if the primary tumor is in this region (thyroid, larynx, or hypopharynx tumors), the low neck is treated exactly as described earlier, and IMRT is used. This has been called the “all in one” technique because all treated regions are being included in one IMRT field.
If the low neck does not contain involved or high-risk regions and if the primary tumor is not located in this region (oropharynx or oral cavity tumors), then an anterior-posterior field is used and matched to the IMRT field. The recommended dose prescription for the low anterior neck field is 50 Gy in 2-Gy fractions. The prescription point is generally ˜3 cm, although this should be individualized based on the depth of the lymph nodes.
The common match point is one slice above the arytenoid cartilages. However, this match line can be moved inferiorly if there are at-risk or involved lymph nodes in this region and an anterior-posterior field is still desired. The acceptable amount of shift before a low anterior neck field is converted into an “all in one” treatment is at the discretion of the physician.
A “cheater” larynx block is placed at the match point, approximately 2 × 2 cm, to avoid excessive spinal cord toxicity in the region of the match line. If the larynx is too close to the planning target volume, such that it cannot be blocked in the AP field, an all-in-one plan should be strongly considered. An example of a low anterior neck field with the spinal cord block is presented in Figure 8.3. Note that two blocks are also drawn parallel to the clavicles to treat the supraclavicular lymph nodes.
Lymph node size is one major criterion for differentiating a lymph node suspicious for disease. In general, a lymph node with a minimal axial diameter of >1.1 cm in the
subdigastric region and >1.0 cm in other nodal regions is considered suspicious for metastasis.7
Other characteristics of a lymph node are also useful. Lymph nodes with a heterogeneous texture or lymph node clusters are suggestive of malignancy. Central hypointensity can also signify infection or inflammation, but in the setting of a primary head and neck cancer, it is highly suspicious for malignant involvement.
Ill-defined borders between a lymph node and surrounding tissue may be indicative of ECE. Although ECE is a pathologic diagnosis, the treating physician may elect to expand the CTV to include the immediate surrounding tissue if ECE is suspected.
The anatomy of a patient with a prior history of head and neck surgery or radiation will typically be more distorted and the tissue planes less well defined. Common postradiation changes include fat stranding, thickening of the larynx, and atrophy of the salivary glands. For a detailed discussion of common postoperative changes, the reader is referred to the study by Som et al.8
It is worth stating that although all pretreatment imaging is “officially” interpreted by a radiologist, there is still an onus on the treating radiation oncologist, particularly in a setting where dose levels are high and margins close, to carefully review the pertinent imaging studies prior to treatment and to verify the relevant findings. In a postoperative patient,
communication with the operating surgeon is vital in determining at-risk regions for the purposes of CTV delineation.
TABLE 8.3 Incidence of Contralateral and Bilateral Neck Node Metastasis by Primary Tumor Site | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
MRI studies contain less artifact (from dental work, metal, etc.) and better soft tissue differentiation than CT scans. Therefore, CT scans are often used for screening, and subsequently, an MRI study is obtained to more definitively determine the extent of disease.
In the absence of MRI availability or in patients with a contraindication to MRI (e.g., pacemaker/defibrillator, cerebral aneurysm clips, severe claustrophobia, inability to tolerate the lengthy examination), a focused CT scan of the head and neck can be of great use in clinically staging a patient’s disease.
In the past decade, multiple studies, both retrospective and prospective in nature, have been performed to assess the
role of PET imaging in head and neck malignancies. The overall conclusions from these studies have been that PET scans are at least as sensitive as CT and MRI in determining the extent of disease and that PET imaging may be of more benefit in detecting residual disease and the need for neck dissection in patients treated with definitive chemoradiation. Selected studies are provided in the following list.
Schwartz et al.9 examined 63 patients staged with either PET/CT or CT scan alone prior to radiotherapy between 2000 and 2003. PET/CT imaging demonstrated a 96% sensitivity and 98.5% specificity for nodal staging. In addition, PET/CT identified nodal disease in two patients thought to have node-negative disease by CT imaging alone, and the correlation between pathologic findings and imaging was stronger when PET imaging was added.
Ng et al.10 examined 134 patients with a clinically negative neck with both [18F]fluorodeoxyglucose ([18F]FDG) PET and CT/MRI. The authors then conducted a visual correlation between the two modalities as compared to histopathologic analysis. The sensitivity of [18F]FDG-PET for nodal metastases was 41.2% compared to 21.6% with MRI. Visual correlation between the two imaging studies was also slightly higher than for PET imaging alone. The probability of occult neck metastases after PET imaging was 6.7% for T1 tumors, 10.8% for T2 tumors, 13.3% for T3 tumors, and 25% for T4 tumors.
Yao et al.11 examined 41 patients treated definitively by radiation who underwent both posttreatment PET/CT imaging and a CT scan alone. Twelve patients had persistent lymphadenopathy and underwent pathologic diagnosis of the enlarged lymph nodes. The pathology did not correlate with pre- or postradiation CT scans but did correlate strongly with the postradiation PET/CT studies. When a maximum standardized uptake value of 3.0 was used, the positive and negative predictive values of PET imaging were 100% and 80%, respectively.
Rogers et al.12 examined 12 patients with stage III or IV cancer of the head and neck who received either posttreatment CT/MRI or PET imaging. Patients then underwent planned neck dissection. Sensitivity and specificity for CT/MRI were 90% and 100%, respectively, whereas for PET imaging, sensitivity and specificity were 45% and 100%, respectively. The authors concluded that PET imaging did not appear to offer any distinct advantage over CT/MRI in the posttreatment setting.
PET and MRI fusion treatment planning is being used in an increasing number of institutions. Although the treating physician should exercise caution in strictly defining the GTV and CTV in correlation with areas of increased FDG uptake, these more sensitive imaging studies can provide useful information in target delineation.
A representation of the nasopharynx is depicted in Figure 8.4. The borders of the nasopharynx are as follows: anterior—continuous with the nasal cavity through the posterior choanae; posterior—level of the first two cervical vertebrae and clivus, made up of four anatomic layers (mucous membrane of pharynx, pharyngeal aponeurosis, superior constrictor muscle of pharynx, and buccopharyngeal fascia); superior—basisphenoid, basiocciput, anterior arch of atlas; inferior—soft palate; and lateral—pharyngeal fascia, including the eustachian tube.
There is significant lymphatic drainage to the nasopharynx, and approximately 85% to 90% of patients with nasopharyngeal cancer have lymph node involvement. Bilateral lymph node involvement occurs in 50% of patients (Table 8.2).2
There are two main routes of lymph node drainage. The first is through the lateral pharyngeal walls to the retropharyngeal nodes (including the node of Rouviere, the most superior retropharyngeal node) and to the subdigastric nodes. The second is a direct route to level V.
Less commonly, the nasopharynx can drain to the parotid gland. This occurs by drainage to the eustachian tube to
the lymph nodes of the tympanic membrane, into the periparotid nodes.
Nasopharyngeal tumors can involve many cranial nerves as they pass into the base of skull, including cranial nerves II to VI and IX to XII. For this reason, it is important to have a comprehensive knowledge of the base of skull. A representative slice is depicted in Figure 8.5.
Most nasopharyngeal carcinomas are of squamous cell origin and are subclassified into (a) squamous cell carcinoma, keratinizing type; (b) nonkeratinizing carcinoma; or (c) undifferentiated carcinoma.
Lymphoepithelial carcinoma is a further subtype that represents nonkeratinizing and undifferentiated carcinomas with an abundance of lymphocytes.
In Asian countries, WHO type III is the most common, while there is a higher relative incidence of WHO type I in the United States.
There are two major categories of symptoms—those that are caused by the extension of the primary tumor and/or lymph node metastases and those that are caused by involved cranial nerves.
Symptoms of local involvement include a neck mass (most frequent symptom), epistaxis, nasal fullness, and headaches.
There are two main patterns of cranial nerve deficits. The first is the petrosphenoidal syndrome of Jacod and involves cranial nerves II to VI through direct extension of the tumor intracranially. Signs of this involvement thus include visual field deficits, facial numbness and/or pain, and ophthalmoplegia.
The second presentation of cranial nerve deficits is the syndrome of the retroparotid space of Villaret. It occurs through involvement of the retropharyngeal lymph nodes with the cranial nerves in the retroparotid space (lymph node levels IX to XII). Signs of this syndrome include dysphagia, difficulties with tongue movement, and trapezius atrophy.
Cranial nerve involvement has been shown to be an important prognostic factor for survival in several studies.13, 14, 15
Less than 5% of patients present with distant metastases. The most common sites of metastases are the liver, lungs, and bone.
Table 8.4 outlines the American Joint Committee on Cancer (AJCC) staging system for nasopharyngeal carcinoma.
Proper staging workup includes:
History and physical, including an endoscopic examination.
Basic laboratory studies, which may include a chest x-ray, CT/MRI scans of the head and neck, and a PET scan. Patients with locally advanced disease or with a suspicion for metastasis may receive a CT scan of the chest/abdomen/pelvis.
Optional molecular studies include Epstein-Barr virus (EBV) serologic tests, including immunoglobulin A (IgA) anti-viral capsule antigen titers (VCA titers).
Nonsurgical management is the primary treatment for nasopharyngeal cancer due to the proximity of the tumor and lymph node metastases to the base of skull.
There have been several retrospective studies examining the effect of conventional radiation therapy alone, with 5-year overall survival rates ranging from 35% to 60%.16, 17, 18, 19, 20
The seminal study regarding general management was the Intergroup Trial by Al-Sarraf et al.21 In this phase III trial, patients with stage III or IV nasopharyngeal cancer were randomized to radiotherapy alone (70 Gy) or radiotherapy with concurrent cisplatin (100 mg/m2) every 3 weeks during treatment, followed by cisplatin (80 mg/m2) and fluorouracil (1,000 mg/m2/day) for 4 days every 4 weeks after the completion of radiation therapy. At 5 years, overall survival rate was 37% in the radiotherapy alone arm versus 67% in the chemoradiation arm, and progression-free survival rate was 29% in the radiotherapy alone arm versus 58% in the chemoradiation arm.22
A more recent study by Wee et al.23 was performed in Singapore, during which 221 patients were randomized to radiation alone (70 Gy in 7 weeks) or radiation plus concurrent cisplatin (weeks 1, 4, and 7 of radiation, 25 mg/m2) followed by adjuvant cisplatin (20 mg/m2) and fluorouracil (1,000 mg/m2) every 4 weeks for three cycles after the completion of radiation therapy. The 3-year overall survival rate was 80% for the chemoradiation arm versus 65% for the radiation alone arm, with a hazard ratio for overall survival of 0.51 (p = 0.0061). This trial confirmed the findings of the Intergroup Trial. This study was of particular significance because, while the Intergroup Trial above included all three histologies, this trial confirmed the above findings in the endemic subtypes (WHO types II and III).
A meta-analysis by Huncharek and Kupelnick24 demonstrated that the addition of chemotherapy to radiation therapy increased both progression-free and overall survival.
A more recent meta-analysis by Langendijk et al.25 examined the benefit of additional neoadjuvant, concurrent, and/or adjuvant chemotherapy to radiation. The authors found an absolute survival benefit of 4% at 5 years. Concomitant chemotherapy provided the largest benefit, with a 20% survival benefit at 5 years.
Based on the inclusion criteria of the two randomized trials above, the standard of care for locally advanced disease (T2b-T4N0 or node-positive disease) is concomitant chemoradiation. For T1-T2aN0 disease, radiation therapy alone is the standard of care.
TABLE 8.4 American Joint Committee on Cancer Sixth Edition Staging for Nasopharyngeal Carcinoma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 8.5 demonstrates the suggested guidelines for target delineation in nasopharyngeal cancer. The gross tumor volume receiving 70 Gy (GTV70) includes the primary tumor and involved lymph nodes.
Due to the high probability of lymph node metastases, levels IB to V and the retropharyngeal lymph nodes should be included in the CTV2 treatment volume bilaterally.
Two dosing regimens are commonly used for the CTV1, CTV2, and CTV3. They are depicted in Table 8.6 and can be applied to other sites of the head and neck being treated with definitive chemoradiation.
In some IMRT protocols, such as RTOG 06-15, described in Section 3.1.7, small-volume lymph nodes can be treated to 63 Gy at the physician’s discretion. However, at Memorial Sloan-Kettering Cancer Center (MSKCC), all high-risk lymph nodes are treated to 70 Gy (CTV1).
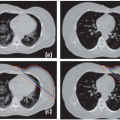
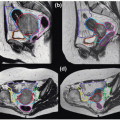
Figure 8.7 depicts sample target volumes for two different patients with locally advanced nasopharyngeal cancer. Note that in all patient examples, for the purposes of wider applicability, the PTVs are depicted. The PTV represents the final treatment volume and is the CTV with an “adequate” margin at the physician’s discretion, as will be described later. It follows that the delineation of PTVs is the same as for the CTV.
Several studies have demonstrated an advantage of IMRT over conventional techniques in the treatment of nasopharyngeal carcinoma.
Kam et al.26 performed a dosimetric comparison of IMRT with two-dimensional (2D) and three-dimensional (3D) conformal radiotherapy in three patients with different stages
of disease (T1N0M0, T2bN2M0, and T4N2M0). The patient with early-stage disease had better parotid gland sparing, whereas the two patients with locally advanced disease had both better tumor coverage and normal tissue sparing.
Xia et al.27 performed another dosimetric study comparing dose conformality, dose-volume histograms, and normal tissue doses with IMRT, 3D conformal methods, and conventional treatment using opposed laterals. The IMRT treatment plans were able to spare normal tissue more effectively while delivering at least 68 Gy to ≥95% of the GTV.
Lee et al.28 reviewed 67 patients who underwent IMRT for nasopharyngeal carcinoma at the University of California-San Francisco between 1995 and 2000. The dosing regimen was 65 to 70 Gy to the CTV1 and 50 to 60 Gy to the CTV2/CTV3. Twenty-six patients also received an intracavitary brachytherapy boost (5 to 7 Gy in two fractions). At a median follow-up of 31 months, only one patient had failed in the primary site and one patient in the neck. The 4-year rate of locoregional progression-free survival was thus 98%. Sixteen patients experienced distant metastases. At 24 months, only one of the 41 assessable patients had grade 2 xerostomia, with the remaining having grade 0 or 1 toxicity.
Kwong et al.29 examined 33 patients treated with IMRT between 2000 and 2002 to a prescribed dose of 68 to 70 Gy. Nineteen of these patients had their salivary flow assessed at baseline and at 2, 6, 12, 18, and 24 months after the completion of radiation. At a median follow-up of 2 years, only one neck failure was observed, such that the 3-year local control and distant metastases-free survival rates were 100%. In addition, the salivary flow continued to increase with time, such that at 2 years, 71% of patients had recovered at least 25% of their baseline stimulated whole salivary flow.
A recent phase II RTOG study examined the outcomes of IMRT to a total dose of 70 Gy with cisplatin if stage T2b-T4 or node positive. The estimated 2-year local control, progression-free, and overall survival rates were 93%, 73%, and 80%, respectively. Acute grade 4 mucositis occurred in 4.4% of patients, and only 2 patients had grade 3 or higher xerostomia 1 year after completing radiation.30
RTOG 06-15 is now accruing patients. This is a phase II study of concurrent chemoradiotherapy using either 3D conformal radiation therapy or IMRT with bevacizumab for locally or regionally advanced nasopharyngeal carcinoma.
TABLE 8.5 Suggested Target Delineation Guidelines for Nasopharyngeal Cancer | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
TABLE 8.6 Dosing Regimens for Definitive Chemoradiation in Head and Neck Cancer | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
The oropharynx consists of four subsites: the soft palate, the palatine tonsillar region (fossa and pillars), the BOT, and the posterior and lateral pharyngeal walls. These are depicted in Figure 8.8, along with the structures of the oral cavity.
The soft palate includes the uvula. It divides the nasopharynx and oropharynx. Anteriorly, it is contiguous with the hard palate and thus divides the oropharynx and oral cavity. Laterally, it attaches to the tonsillar pillars. Tumors more often arise from the inferior aspect of the soft palate than the superior aspect.
The palatine tonsils include the tonsillar fossa and tonsillar pillars. Within the tonsillar pillars are the palatoglossus and palatopharyngeus muscles. Inferiorly, the tonsillar fossa becomes the glossopalatine sulcus. Superomedially, the tonsils are connected to the soft palate.
The BOT is bounded anteriorly by the circumvallate papilla and posteriorly by the vallecula (although the vallecula is considered a part of the BOT). Its lateral borders are the glossopalatine sulci.
The posterior pharyngeal wall is composed of the pharyngeal constrictor muscles. It consists of multiple layers: the mucosa, submucosa, pharyngobasilar fascia, superior constrictor muscle, and buccopharyngeal fascia. It is bounded superiorly by the region of the soft palate and inferiorly by
the epiglottis. The lateral pharyngeal wall extends from the pharyngoepiglottic fold to the piriform sinus.
The oropharynx has a rich lymphatic network and primarily drains into the subdigastric, upper cervical (II and III), and retropharyngeal lymph nodes (in proximity to cranial nerves IX to XII).
Progression of nodal metastases is usually orderly, starting at level II and proceeding inferiorly to levels III and IV. Skip nodal metastases are relatively rare.
Table 8.2 gives an overview of the risk of lymph node metastases by lymph node level,2 which has also been described by Lindberg.31
The incidence of ipsilateral lymph node metastasis is 80% BOT and tonsil, 60% oropharyngeal wall, and 45% anterior pillar and soft palate.
The incidence of contralateral lymph node metastasis is 30% BOT, 15% soft palate, and 10% tonsil.
 Figure 8.8. Structures of oral cavity and oropharynx. (From National Cancer Institute. SEER Training Modules. http://training. seer.cancer.gov/, with permission.) |
TABLE 8.7 American Joint Committee on Cancer Sixth Edition Staging for Tumors of the Oropharynx | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The vast majority of tumors of the oropharynx are squamous cell carcinomas (>90%).
The most common initial sign is an asymptomatic neck mass.
Other common symptoms include otalgia, odynophagia, dysphagia, changes in voice, and foul breath.
Otalgia is caused by involvement of cranial nerves IX and X.
With cranial nerve IX involvement, the patient experiences deep ear pain. The pathway is through the petrosal ganglion to the tympanic nerve of Jacobson.
With cranial nerve X involvement, pain is localized to the pinna and the middle ear. The pathway is through the auricular nerve of Arnold.
On physical examination, possible signs include trismus, impairment of tongue protrusion, and vocal cord impairment.
Table 8.7 outlines the AJCC staging system for carcinoma of the oropharynx.
The staging evaluation should include the following:
History and physical, to include fiberoptic examination.
Routine laboratory studies.
Pathologic sampling, which may include fine-needle aspiration (FNA) or excisional biopsy of suspicious lymph nodes and FNA of suspicious oropharyngeal lesions.
Extent of disease imaging, which may include CT/MRI of the head and neck, PET scan, and chest x-ray (if stage T3/T4 or N2/N3, a chest CT should be obtained).
Surgery and adjuvant radiation with or without chemotherapy was previously the treatment paradigm.
The RTOG 73-03 study (Kramer et al.32) was the first to suggest that surgery was not necessary as a component of treatment. This study included a three-arm trial of oral cavity and oropharynx cancer examining preoperative radiotherapy (5,000 cGy), postoperative radiotherapy (6,000 cGy), and definitive radiation therapy (6,500-7,000 cGy), reserving surgery for salvage. Although a small number of patients were included, the authors found no significant difference in overall survival or locoregional control. The authors concluded that for these sites, the use of definitive radiation with surgical salvage was a question for future research.
Parsons et al.33 compiled results from 11 institutions from 1970 to 2000 using a MEDLINE search to determine if there was a difference in outcomes for patients treated with surgery with or without adjuvant radiation versus definitive radiation with or without neck dissection. Although rates of locoregional control, 5-year overall survival, and 5-year cause-specific survival were similar in the two groups, the rate of significant complications was higher in the surgery with or without adjuvant radiation group. The authors concluded that a nonsurgical approach was favored.
A subsequent study assessed the optimal fractionation regimen for local control. Fu et al.34 (90-03) performed a randomized trial with 1,073 patients with locally advanced head and neck cancer, randomizing them to (a) standard fractionation at 2 Gy once daily to 70 Gy; (b) accelerated fractionation (1.2 Gy twice a day [BID] to 81.6 Gy); (c) accelerated fractionation with a split course (1.6 Gy BID to 38.4 Gy, then a 2-week break, then to 67.2 Gy); or (d) accelerated fractionation with a concomitant boost (1.8 Gy daily to 72 Gy, with a boost of 1.5 Gy as a second daily treatment for the last 12 fractions). Arms (b) and (d) had better locoregional control than arms (a) and (c). In addition, Bourhis et al.35 performed a meta-analysis in 2006 comparing conventional radiotherapy with hyperfractionated or accelerated radiotherapy, or both, in patients with non-metastatic head and neck cancer (74% with oropharynx/larynx cancer). The authors found an overall survival benefit of 3.4% at 5 years with altered fractionation, and an even larger benefit in the subset of patients receiving hyperfractionation (8% at 5 years).
With the advent of IMRT, an analogous fractionation is being added to the concomitant boost, with various dose distributions at different levels (CTV1, CTV2, and CTV3).
Multiple trials have been performed to identify patients who would benefit most from a combined chemotherapeutic regimen, determine what the optimal timing should be, and assess whether the addition of chemotherapy can compensate for less aggressive radiation regimens in terms of efficacy.
Denis et al.,36 in RTOG 94-01, randomized 226 patients with stage III or IV oropharyngeal carcinoma to either radiation alone (70 Gy in 2-Gy fractions) or concomitant chemoradiation with the same radiation regimen plus carboplatin (70 mg/m2) with fluorouracil (600 mg/m2). Five-year overall survival (22% for chemoradiation vs. 16% for radiation alone), disease-free survival (27% for chemoradiation vs. 15% for radiation alone), and locoregional control (48% for chemoradiation vs. 25% for radiation alone) all favored the Note that because 85% of these patients had stage T3 or T4 tumors, the proportion of surviving patients was less than in other studies.
Pignon et al.37 performed a meta-analysis that included trials between 1965 and 1993 of patients with carcinoma of the oropharynx, oral cavity, larynx, or hypopharynx. In the subanalysis of six trials comparing neoadjuvant versus concomitant or alternating chemoradiation, there was an overall survival benefit of 4% for chemotherapy overall, and 8% for concomitant chemotherapy.
Adelstein et al.38 randomized 295 patients with stage III or IV head and neck cancer to (a) radiation alone (70 Gy in two fractions); (b) radiation (70 Gy in two fractions) with cisplatin (100 mg/m2) on days 1, 22, and 43; or (c) split-course radiation therapy (30 Gy plus 30 to 40 Gy, for a total dose of 60 to 70 Gy) with three courses of continuousinfusion fluorouracil (1,000 mg/m2/day) and cisplatin (75 mg/m2). Arm (b) had a better 3-year projected overall survival than arms (a) and (c) (37% vs. 23% vs. 30%, respectively; p = 0.014). The authors concluded that chemotherapy could not be used to compensate for the loss of efficacy produced by split-course radiation, as compared to more accelerated fractionation regimens.
Therefore, we advocate the management paradigm in Table 8.8 for patients with oropharyngeal carcinoma.
Table 8.9 depicts suggested guidelines for target delineation in oropharyngeal carcinoma, as well as the recommended dosing regimen, derived from Chao et al.2 Table 8.6 provides an alternative dosing regimen for these tumors.
Note that the bilateral neck is covered in all oropharyngeal lesions other than T1N0 and select T2N0 tonsillar lesions. However, if the lesion is not well lateralized, then bilateral neck coverage could be considered in these situations as well.
Although less common, some patients undergo surgical intervention (either for small-volume disease or extensive resection for locally advanced disease) as primary treatment for oropharyngeal carcinoma. Table 8.10 provides two recommended dosing regimens for patients being treated with radiation in the postoperative setting.
Following the guidelines from Table 8.10, if a patient is being treated in the postoperative setting with negative margins, the tumor bed and the high-risk lymph nodes should be included in the high-dose region (CTV1), and the low-risk lymph nodes should be included in the CTV3 region. In a patient with positive margins, the tumor bed region at highest risk can be included in the CTV1, with the CTV2 and CTV3 following the same delineation guidelines as for definitive treatment.
Figures 8.9, 8.10, and 8.11 depict the delineation of representative patients from MSKCC.
TABLE 8.8 Management Strategy for Oropharyngeal Carcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|
|
TABLE 8.9 Suggested Target Delineation Guidelines for Oropharyngeal Carcinoma | |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree