The Middle Ear and Mastoid
Anatomy and Normal Variations
Temporal Bone
The temporal bone consists of five definable segments: squamous, petrous, tympanic, mastoid, and styloid (Fig. 3.1).1,2 The bulk of the external surface of temporal bone is comprised of the squamous portion, which has horizontal and vertical components separated by the zygoma. The squamous portion articulates with the occipital, parietal, and sphenoid bones and forms part of the lateral wall of the middle cranial fossa and the medial wall of the temporal fossa. The zygomatic process forms the roof of the temporomandibular fossa (TMJ). The external surface of the temporal squama contains a sulcus for the superficial temporal branch of the external carotid artery. The internal surface contains a sulcus for the superior petrosal sinus. The petrosquamous suture (a portion of which is made up of Koerner’s septum) separates the squamous from the petrous temporal bone. The petrous portion is a quadrangular pyramid that forms the bulk of the internal surface of the temporal bone, extends to the petrous apex, and contains the inner ear structures (vestibule, semicircular canals, cochlea) and virtually all major neurovascular compartments (internal auditory canal [IAC], carotid canal, jugular fossa). The apex of the pyramid rests on the clivus at the petrooccipital fissure. The tympanic bone makes up the bulk of the external auditory canal (EAC) and middle ear space. It is separated anteriorly and internally from the petrous bone by the petrotympanic (glaserian) fissure and anteriorly and externally from the squamous portion by the tympanosquamous suture. The mastoid portion contains the mastoid air cell system, articulates laterally with the parietal and occipital bones, and communicates with the nasopharynx via the eustachian tube. There is contiguity of the mastoid air cells and those of the petrous pyramid (petrous apex). The mastoid process forms a bony protuberance in the retroauricular region and is not ossified at birth. There is also a rudimentary styloid portion of the temporal bone, which originates in cartilage (second branchial arch) and makes up the posterior tympanum and styloid process. The temporal bone forms a portion of the floor of the middle and posterior cranial fossae (Table 3.1 and Table 3.2).
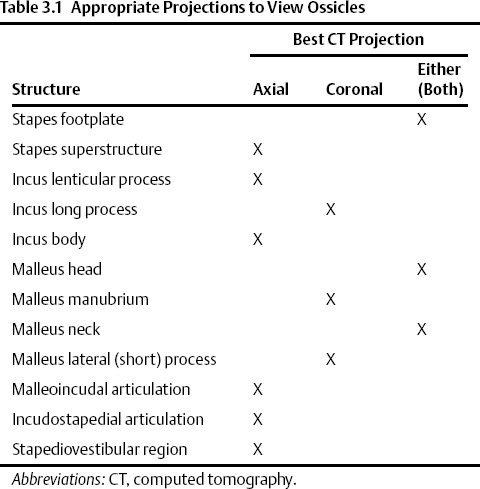
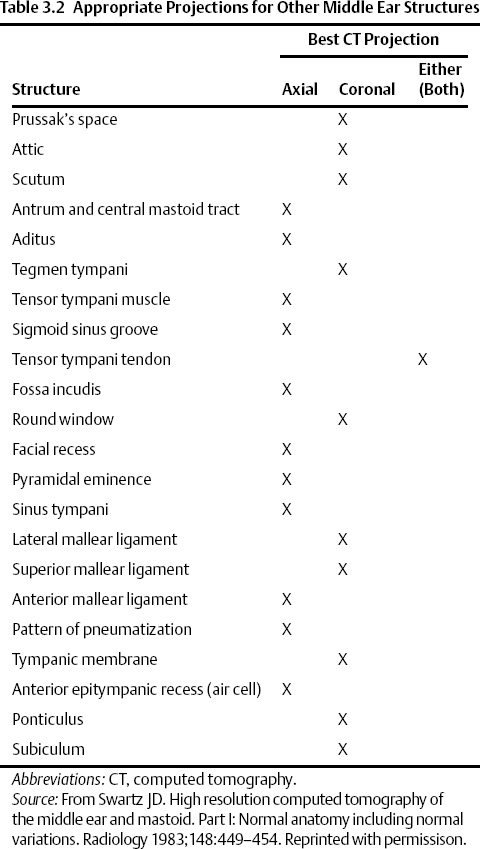
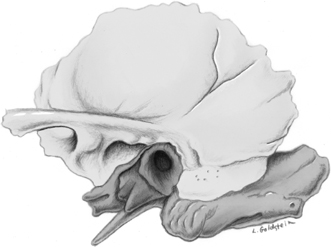
Fig. 3.1 Temporal bone–external surface (yellow, squamous; orange, mastoid; blue, tympanic; green, styloid). The petrous portion is predominantly deep to the illustration; a segment is in orange anterior to the tympanic portion. (Adapted from Platzer W. Pernkopf Anatomy, 3rd ed. Munich: Urban & Schwarzenberg; 1989.) (See Color Plate Fig. 3.1.)
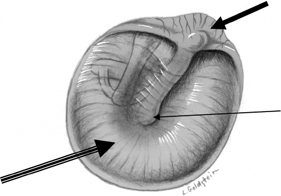
Fig. 3.2 Tympanic membrane (solid arrow, pars flaccida; outlined arrow, pars tensa; long thin arrow, umbo [malleus handle]). (Adapted from Platzer W. Pernkopf Anatomy, 3rd ed. Munich: Urban & Schwarzenberg; 1989.) (See Color Plate Fig. 3.2.)
Tympanic Membrane
The tympanic membrane (TM) is 1 mm thick and separates the EAC from the middle ear (mesotympanum) (Fig. 3.2). The sites of attachment (tympanic annuli) are well seen on both axial and coronal computed tomography (CT) projections (see Chapter 2. 3,4 The elliptical or cone shape corresponds to the contour of this portion of the canal. In adults, it is angulated ∼ 140 degrees with respect to the superior border of the EAC. Standard measurements are 1 cm vertically and 9 mm horizontally.5–7 The handle (manubrium) and lateral (short) process of the malleus are embedded in the TM, forming the umbo and malleal prominence, respectively. Folds extending from the malleal prominence to the anterior and posterior tympanic spines (anterior and posterior malleal plicae) separate the TM into a smaller, lax, but thicker pars flaccida above and a larger, taut, more fibrous pars tensa below.8–10 The pars tensa and pars flaccida both contain three layers, an external epidermal (squamous) layer continuous with the skin lining the EAC (ectoderm), an inner layer (mucosal) continuous with the middle ear mucosa (endoderm), and an intermediate fibrous layer (mesoderm).11,12 The central fibrous (mesodermal) layer of the pars flaccida is less well developed but contains more elastic fibers. A healing perforation of the pars tensa may fail to reform this fibrous layer and is more likely to retract. The normal pars tensa can be seen on axial and coronal CT using appropriate windows and levels, and its location can be further inferred from the position of the manubrium of the malleus on coronal images (Fig. 3.3 and Fig. 3.4). A thickened or significantly retracted pars tensa is easily appreciated (see below).4 Simple perforations themselves are difficult to identify; however, this is an inconsequential point because they are so easily visualized otoscopically. The pars flaccida is consistently seen on coronal sections in the normal patient, extending from the lateral process of the malleus to the scutum. Simple retractions of this segment are also possible to discern with CT although they are also much more easily evaluated otoscopically.13 Innervation of the TM is via contributions from the mandibular nerve (V3), Arnold’s nerve (vagus), and Jacobson’s nerve (glossopharyngeal). This complex arrangement partially explains the clinical difficulties in differentiating referred otalgia from primary causes of ear pain.14,15
Ossicles, Suspensory Ligaments, and Tendons
The normal ossicular chain consists of the malleus, incus, and stapes (Fig. 3.5). The stapes weighs only 2.5 g and as such is only about one tenth the weight of either of the other two ossicles.1 A review of paleontology reveals that the precursors of the ossicular chain were part of the jaw and that primitive vertebrates such as the bullfrog have only one middle ear bone.16 The development of the ossicular chain was presumably a survival mechanism, as it amplifies sound pressure on the TM by 30%. A primitive stapes (solid, no crura) persists in various marsupials. The development of crura improved hearing, as the resultant ossicle is much lighter. The tympano-ossicular system is responsible for transmission of sound from the EAC to the cochlea in the normally functioning ear. This is referred to as ossicular coupling. Direct stimulation of the oval and round windows in those with a nonfunctioning ossicular chain is referred to as acoustic coupling.17
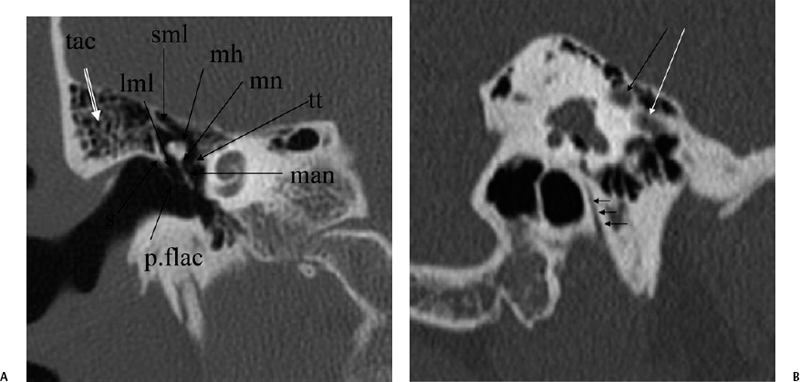
Fig. 3.3 (A) Coronal CT anatomy (sml, superior malleal ligament; mh, malleus head; mn, malleus neck; man malleus, manubrium; s, scutum; p.flac, pars flaccida of tympanic membrane; lml, lateral malleal ligament; tt, tensor tympani muscle and tendon; tac, tegmental air cells [above external auditory canal]; the lml and the p.flac. subtend Prussak’s space). (B) Sagittal CT image; long white arrow, facial nerve canal (labyrinthine segment); long black arrow, cochleariform process (tensor tympani muscle); triple black arrows, inferior tympanic canaliculus (nerve of Jacobson).
The malleus is described in terms of the head, neck, lateral (short) process, anterior process, and handle (manubrium) (Fig. 3.6, Fig. 3.7, Fig. 3.8). The lateral process and manubrium are embedded within the TM and are best seen utilizing coronal CT images.4,18 There is a diarthrodial articulation between the malleus and incus in the attic, the malleoincudal articulation, easily and consistently seen on axial and sagittal CT images (Fig. 3.9 and Fig. 3.10).6,19 There are medial and lateral incudomallear ligaments that are difficult to resolve even with the highest resolution CT equipment.19 The bulk of the incus, the largest ossicle, is made up of the body; however, short, long, and lenticular processes are also described (Figs. 3.6, 3.7, 3.9, 3.10). The short process lies posteriorly within the fossa incudus and acts as a fulcrum on which the rest of the incus rotates. The fossa incudus is located immediately below the aditus and can only be appreciated with axial and sagittal CT sections.4,10 Surgeons are aware of the close relationship between the short process and the second genu of the facial nerve, generally in the 3 mm range.20 The very fine long process and lenticular process represent the most vulnerable segments of the ossicular chain and are commonly eroded in the context of inflammatory disease.7,21,22 They meet at a variable angle, usually almost 90 degrees (Fig. 3.11 and Fig. 3.12). The long process is visualized to best advantage on these coronal CT sections. The cup-shaped lenticular process articulates directly with the ball-shaped capitulum (head) of the stapes via a cartilaginous disk and is also a synovial, diarthrodial articulation (Fig. 3.13, Fig. 3.14, Fig. 3.15, Fig. 3.16, and Fig. 3.17).11,23–25 The stapes super-structure is a term that is used to describe the portion of the stapes that is derived from the second branchial arch. This includes the capitulum (head), anterior crus, posterior crus, and the tympanic portion of the footplate. The vestibular portion of the footplate and contiguous annular in the annular ligament that supports the syndesmotic (fibrous) stapediovestibular articulation (Fig. 3.13D and Fig. 3.16B).
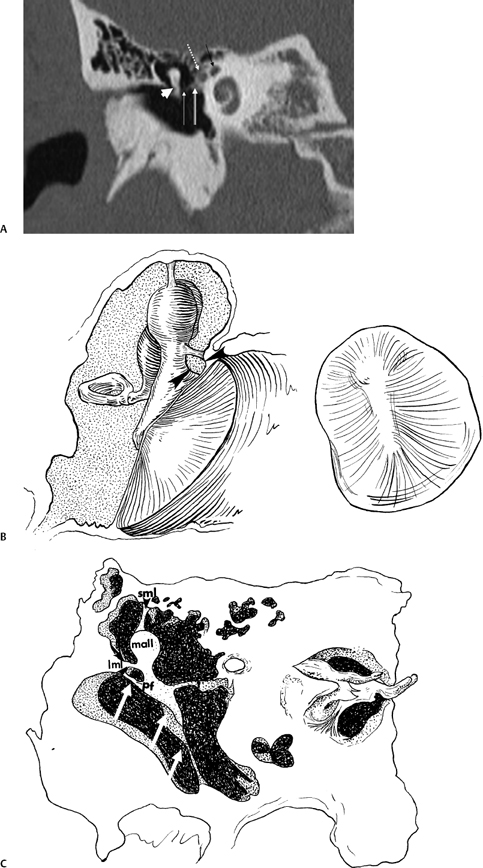
Fig. 3.4 (A) Short black arrow, facial nerve (labyrinthine segment); dotted white arrow, facial nerve (tympanic segment); cross-hatched white arrow, tensor tympani muscle; thin white arrow, tensor tympani tendon; thick white arrowhead, malleus neck. (B) Artist’s rendering of Prussak’s space (arrowheads). This is the space subtended by the lateral mallear ligament, the malleus neck, and the pars flaccida of the tympanic membrane. Insert: Otoscopic view of normal tympanic membrane. (C) Drawing, coronal plane (lml, lateral mallear ligament; sml, superior mallear ligament; mall, head of malleus). White arrow, pars flaccida of tympanic membrane (pf); double white arrows (pars tensa of tympanic membrane).
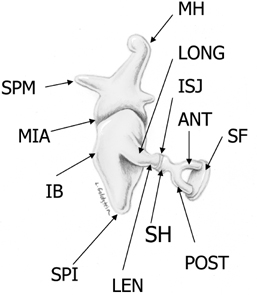
Fig. 3.5 Ossicular chain (SPM, malleus-short process; MH, malleus handle; MIA, malleoincudal articulation; IB, incus body; SPI, incus, short process; LONG, incus, long process; LEN, incus, lenticular process; ISJ, incudostapedial joint; SH, stapes head; ANT, stapes, anterior crus; POST, stapes, posterior crus; SF, stapes footplate [note tympanic and vestibular segments]). (See Color Plate Fig. 3.5.)
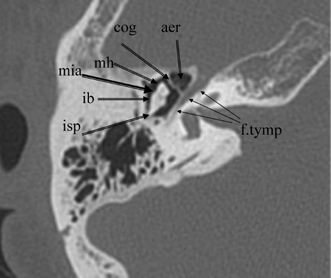
Fig. 3.6 Axial anatomy (isp, incus short process; ib, incus body; mh, malleus head; cog, cog; aer, anterior epitympanic recess [single cell]; f. tymp, facial nerve tympanic segment; mia, malleoincudal articulation).
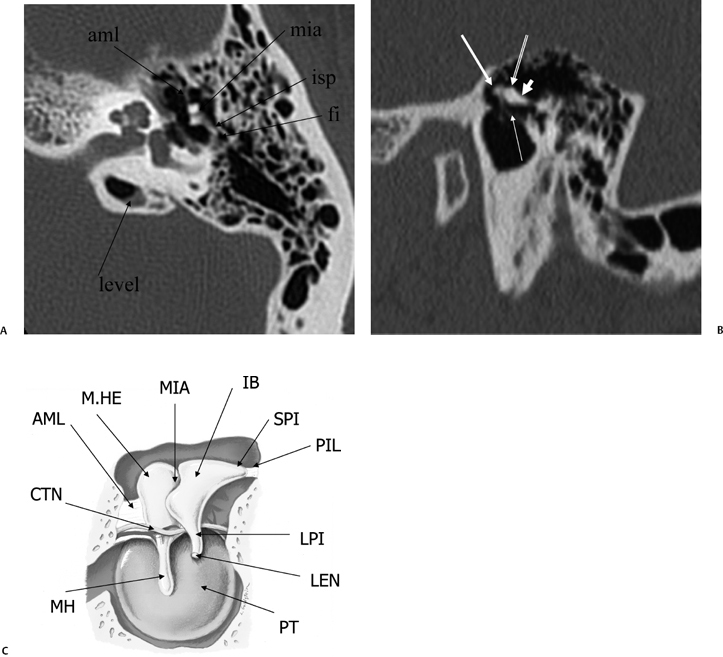
Fig. 3.7 (A) Axial anatomy (aml, anterior malleal ligament; mia, malleoincudal articulation; isp, incus, short process [in fossa incudus]; fi, fossa incudus). Level-trapped fluid in petrous apex cell. (B) Sagittal view. White arrow, malleus head; outlined white arrow, incus body; short, thick white arrow, incus short process; thin white arrow, tympanic membrane (pars flaccida). (C) Drawing, sagittal plane; tympanic cavity (AML, anterior malleal ligament; CTN, chorda tympani nerve; M.HE, malleus head; MH, malleus handle; MIA, malleoincudal articulation; IB, incus body; SPI, Incus, short process; PIL, posterior incudal ligament; LPI, Incus, long process; LEN, incus, lenticular process [stapes is removed]; PT, pars tensa, tympanic membrane). (See Color Plate Fig. 3.7C.)
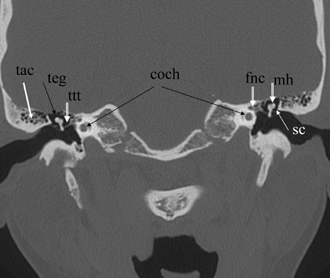
Fig. 3.8 Coronal computed tomography image, well positioned. Bothears are appreciated in a symmetric fashion due to superb patientpositioning (mild tilt; teg, tegmen tympani [roof of middle ear/attic];mall, malleus head; tac, tegmental air cells [variable in number]; sc,scutum; ttt, tensor tympani tendon [5th nerve, 1st branchial arch derivation]; fnc, facial nerve canal; coch, apical/middle cochlear turns).
Contrary to popular belief, the vast bulk of the ossicular chain is best appreciated on evaluation of axial CT sections. The malleoincudal and incudostapedial articulations as well as the stapes superstructure are all appreciated to best advantage in this projection.4 The oval window (stapes footplate/annular ligament) is of uniform thickness and has an anteroposterior orientation. In our opinion this structure is also best seen in this projection (Fig. 3.15).26,27 Coronal CT images allow for better appreciation of structures oriented vertically, such as the malleus and incus long process. The right-angle junction of the incus long and lenticular processes is also well appreciated in this projection. Axial, coronal, and sagittal CT images are therefore highly complementary for ossicular evaluation (Fig. 3.11 and Fig. 3.12).28,29
Sagittal CT imaging is more readily available with current techniques utilizing volumetric acquisitions (see Chapter 1). In this projection, the malleoincudal articulation is well seen as the classic “molar tooth” configuration (Fig. 3.7C and Fig. 3.9B). This appearance was originally described with complex motion tomography when direct sagittal (lateral) imaging was routine. Other structures visualized in this projection include the recess for the stapedius muscle, the posterior semicircular canal, and the anterior tympanic spine (Fig. 3.9B, Fig. 3.11B,C, and Fig. 3.17B–D). The latter forms the undersurface of the glaserian (anterior tympanic) fissure and is a common fracture site.
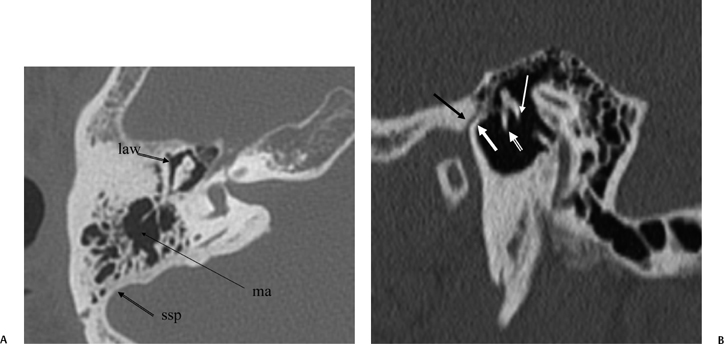
Fig. 3.9 (A) Axial anatomy (law, lateral attic wall; ma, mastoid antrum;ssp, sigmoid sinus plate). (B) Sagittal computed tomography image.Thin white arrow, long process of incus; outlined white arrow, handle of malleus (note the classic “molar tooth” appearance); thick white arrow, anterior tympanic spine; black arrow, glaserian fissure (passage of chorda tympani nerve and anterior tympanic artery).
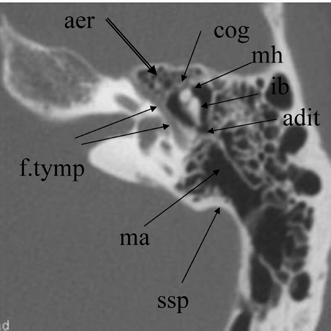
Fig. 3.10 Axial anatomy (aer, anterior epitympanic recess [multiple cells]; cog, cog; mh, malleus head; ib, incus body; adit, aditus ad antrum; ma, mastoid antrum; ssp, sigmoid sinus plate; f.tymp, facial nerve, tympanic segment).
The malleus is supported by superior, anterior, and lateral mallear ligaments. These structures are well seen on a careful study of the CT scan.4 The superior and lateral ligaments are seen best on coronal sections and the anterior ligament best on axial sections (Fig. 3.4, Fig. 3.7, Fig. 3.13). A posterior incudal ligament exists; however, it is thin and not visualized on CT (Fig. 3.7C). The incus is therefore quite poorly supported, particularly distally (see Chapter 6).11,30–32
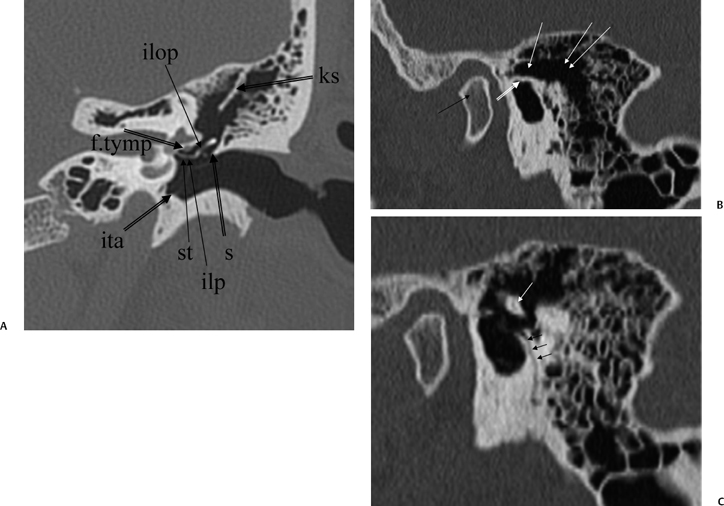
Fig. 3.11 (A) Coronal anatomy (ks, Koerner septum; ilp, incus, lenticular process; ilop, incus long process; st, stapes; s, scutum; f.tymp, facial nerve canal, tympanic segment; ita, inferior tympanic annulus [attachment of tympanic membrane]). (B) Sagittal image, more lateral. Black arrow, mandibular condyle; outlined white arrow, scutum; white arrow, attic (lateral to ossicular mass); double white arrows, mastoid antrum. (C) Sagittal image, more medial. Triple black arrows, canaliculus chordae tympani; white arrow, incus body.
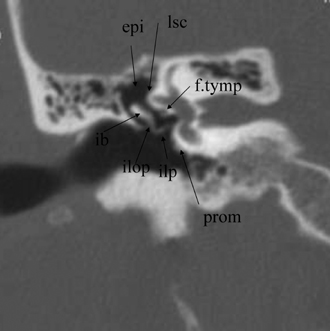
Fig. 3.12 Coronal anatomy (epi, epitympanum; lsc, lateral semicircular canal; prom, promontory [basilar turn of cochlea]; ilop, incus, long process; ilp, incus, lenticular process; ib, incus body; f.tymp, facial nerve canal, tympanic segment).
Two muscles, the tensor tympani, which is a first (Meckel) branchial arch derivative innervated by the fifth cranial nerve (CN V), and the stapedius, which is a second (Reichert) branchial arch derivative innervated by the CN VII, also participate in ossicular support. The tensor tympani muscle lies within a narrow bony channel (semicanal) parallel and medial to the eustachian tube.6,7,33,34 The tendon of this muscle continues to course posterolaterally until it reaches a spoon-shaped depression adjacent to the cochlea referred to as the cochleariform process. The latter is an important surgical landmark indicating proximity to the facial nerve canal. From here, the tendon courses laterally to reach the neck of the malleus. The tendon is easily visualized on both coronal and axial CT sections due to its mediolateral orientation (Fig. 3.8, Fig. 3.13, and Fig. 3.16). The stapedius muscle travels in a bony sulcus just medial to the second genu of the facial canal. It emerges from the pyramidal eminence and courses anteriorly to attach to the stapes anywhere from the incudostapedial region to the junction of the posterior crus with the footplate. It can only be appreciated on axial CT sections (Fig. 3.16). The tensor tendon tightens the TM, and the stapedius tendon stretches the annular ligament. As such, these muscles both play a role in damping the response of the ossicular chain, thus protecting the cochlea from intense acoustic stimulation.24,35
Recesses and Ridges
The tympanic cavity contains numerous recesses and ridges.36 Most notable among these recesses is the superior recess of the TM, better known as Prussak’s space, which is bordered laterally by pars flaccida, inferiorly by the lateral (short) process of the malleus, superiorly by the lateral mallear ligament, and medially by the neck of the malleus (Fig. 3.4, Fig. 3.8). 4,9,36,37 The lateral mallear ligament courses from the scutum (junction of the lateral attic wall and EAC) to the neck of the malleus. Prussak’s space opens posteriorly into the epitympanum and represents the most common site of origin for acquired attic cholesteatoma (CH).
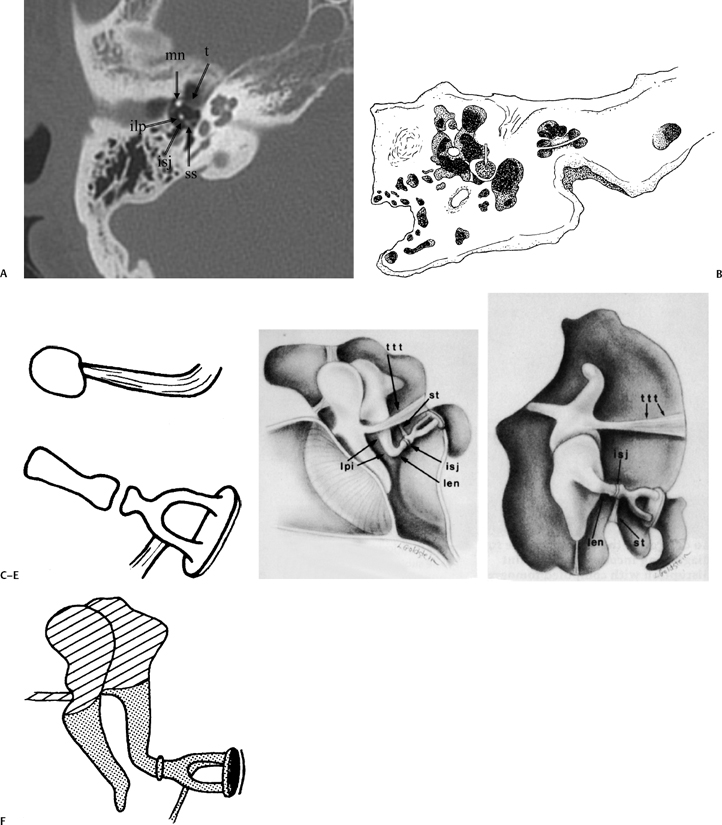
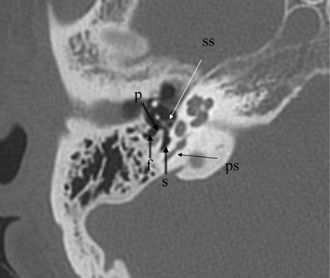
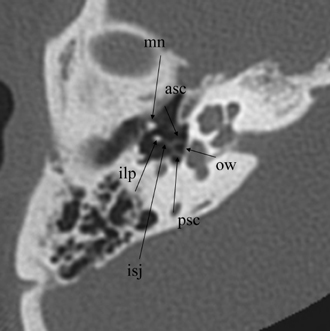
Fig. 3.15 Axial anatomy (mn, malleus neck; asc, anterior stapes crus; psc, posterior stapes crus; ilp, incus, lenticular process; isj, incudostapedial joint; ow, oval window [stapes footplate/annular ligament]).
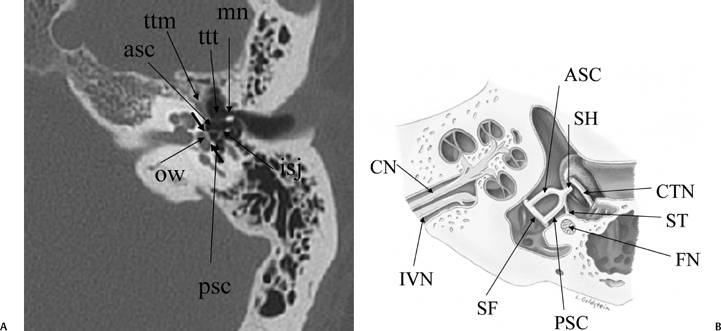
Fig. 3.16 (A) Axial anatomy (ttm, tensor tympani muscle; ttt, tensor tympani tendon; mn, malleus neck; ow, oval window [small unlabeled arrows, anterior and posterior margins]; asc, anterior stapes crus; psc, posterior stapes crus; isj, incudostapedial joint). (B) Tympanic cavity, corresponding axial illustration (CTN, chorda tympani nerve; SH, stapes head; ASC, stapes, anterior crus; PSC, stapes, posterior crus; SF, stapes footplate [note tympanic and vestibular segments]; ST, stapedius tendon; FN, facial nerve, second genu in pyramidal eminence; CN, cochlear nerve; IVN, inferior vestibular nerve). (See Color Plate Fig. 3.16B.)
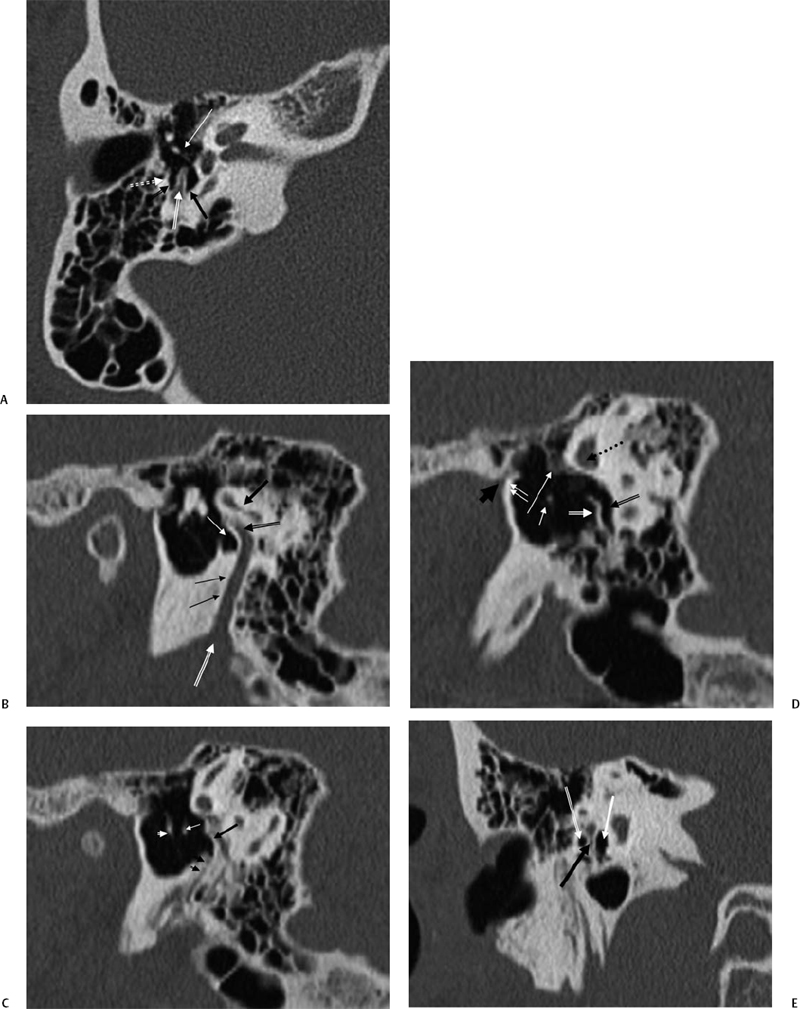
Fig. 3.17 (A) Posterior tympanum. Outlined black arrow, facial recess; outlined white arrow, pyramidal eminence; black arrow, sinus tympani; white arrow, incudostapedial articulation; dotted white arrow, chordal eminence. (B) Sagittal computed tomography (CT) image, more lateral. Black arrow, lateral semicircular canal; outlined black arrow, facial nerve canal, second genu; thin white arrow, sinus tympani; double black arrows, facial nerve canal (mastoid segment); outlined white arrow, stylomastoid foramen. (C) Sagittal CT image, more medial. Black arrow, pyramidal eminence; double black arrowheads, stapedius muscle canal; white arrow, incus lenticular process; white arrowhead, malleus neck. (D) Sagittal CT image, most medial. Outlined white arrow, subiculum; outlined black arrow, sinus tympani; dotted black arrow, lateral semicircular canal; long white arrow, facial nerve (tympanic segment); short white arrow, stapedial head; double white arrows, anterior tympanic spine; short thick black arrow, glaserian fissure (exit of chorda tympani nerve). (E) Posterior tympanum, coronal CT image. Outlined white arrow, facial recess; thick black arrow, pyramidal eminence; thick white arrow, sinus tympani.
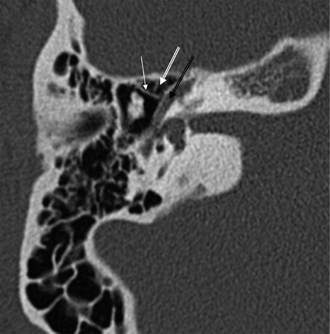
Fig. 3.18 Anterior epitympanic recess. Outlined white arrow, anterior epitympanic recess (supratubal recess); thin white arrow, cog; outlined black arrow, facial nerve/canal (tympanic segment).
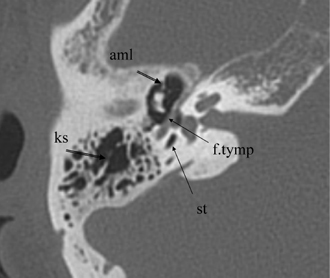
Fig. 3.19 Axial anatomy (aml, anterior malleal ligament; ks, Koerner septum; st, sinus tympani; f.tymp, facial nerve canal, tympanic segment).
The anterior epitympanic recess (AER), also known as the supratubal recess (STR), is located superior to the bony eustachian tube and anterior to the attic and consists of a variably sized single air cell (61%) or multiple small cells. There is symmetry in 78% of patients.38 This region is visualized on axial section anteromedial to the head of the malleus. It is bounded posteriorly by a thin transverse bony septum (“cog”) and anteriorly by the anterior petrosal tegmen (Fig. 3.6, Fig. 3.10, and Fig. 3.18). The middle cranial fossa forms a portion of the superior and anterior boundary. The chorda tympani nerve and the tympanic bone form the lateral boundary.39 The “cog” may be bony or fibrous and extends from the cochleariform process to the tegmen.40 The shape of this recess is determined by the embryologic development of the saccus anticus and anterior saccule of the saccus medius41; however, development of the AER/STR is independent of the middle ear/mastoid air cells system, instead relating directly to eustachian tube formation.42 Growth of the AER/STR may continue into early childhood in contradistinction to the remainder of the attic, which is generally believed to be complete by the end of gestation. The proximal tympanic segment of the facial nerve canal lies immediately adjacent to the recess on its medial side (Fig. 3.10 and Fig. 3.18). The mucosal fold investing the tensor tympani is also in close apposition.4,43,44 When this fold is embryologically absent, cholesteatomatous masses have direct access to this segment of the facial nerve.45 Koerner’s septum (see below) is the posterior continuation of the “cog” and therefore has similar embryologic significance (Fig. 3.19).46
A complex set of recesses and ridges lies posterior to the bony tympanic annulus along the posterior and medial borders of the tympanic cavity. This region is referred to in the literature as the posterior tympanum or retrotympanum (Fig. 3.14, Fig. 3.17, Fig. 3.20, Fig. 3.21, Fig. 3.22, Fig. 3.23, and Fig. 3.24).36,47 The pyramidal eminence (PE), from which the stapedius tendon emerges, represents the most prominent ridge in the posterior wall. Immediately medial to the PE lies the sinus tympani, a recess of variable depth that is bordered medially by the cortical bone overlying the posterior semicircular canal.12,36,47 The ponticulus (an extension of the oval window niche) is its superior border; the subiculum forms the inferior border (Fig. 3.20A). The subiculum separates the sinus tympani from the round window niche (Fig. 3.23).4,48 Directly lateral to the PE lies the facial recess, which is often much shallower than its more medial counterpart. Lateral to the facial recess is the chordal eminence, which forms the medial border of the canaliculus chordae tympani through which the chorda tympani branch of the facial nerve enters the middle ear cavity. A chordal ridge is described that links the pyramidal eminence to the chordal eminence. The facial recess is limited further laterally by the bony tympanic annulus (origin of the TM).
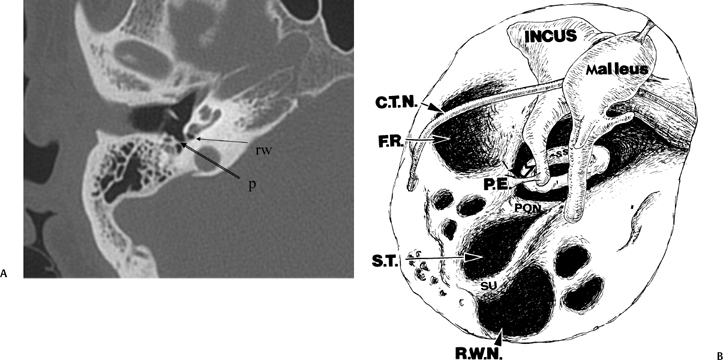
Fig. 3.20 (A) Axial anatomy (p, ponticulus; rw, round window niche). (B) Artist’s rendering of posterior tympanum [C.T.N., chorda tympani nerve (arrow); F.R., facial recess (arrow); S.T., sinus tympani; SU, subiculum; R.W.N., round window niche (arrowhead); PON, ponticulus; P.E., pyramidal eminence (open arrowhead); stapedius tendon (arrow); SS, stapes superstructure].
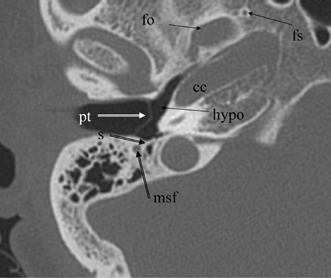
Fig. 3.21 Axial anatomy (pt, pars tensa, tympanic membrane; s, subiculum; f.mast, facial nerve canal, mastoid segment; hypo, hypotympanum; fo, foramen ovale; fs, foramen spinosum; cc, carotid canal).
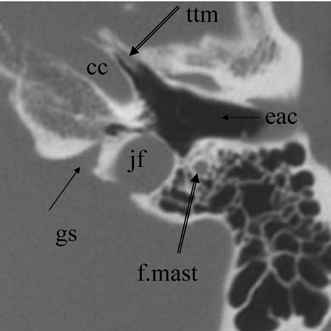
Fig. 3.22 Axial anatomy (ttm, tensor tympani muscle; cc, carotid canal, horizontal portion; f.mast, facial nerve canal, mastoid segment; jf, jugular foramen; eac, external auditory canal; gs, glossopharyngeal sulcus).
The medial wall of the posterior tympanum is described as having two ridges and three depressions (Fig. 3.14, Fig. 3.20, Fig. 3.23, and Fig. 3.24). The ridges are the more inferior subiculum (posterior prolongation of the cephalad border of the round window) and the more superior ponticulus, which extends from the pyramidal eminence to the promontory. Between these ridges lies the sinus tympani.12 Beneath the subiculum is the round window niche, and superior to the ponticulus is the oval window. These ridges are variable in size. They are identified with difficulty on coronal section; however, their importance is limited to both the surgeon and the radiologist. Two additional eminences, the styloid and the chordal, and two other depressions, the posterior and lateral tympanic sinuses, are also probably identifiable on CT; however, their importance is limited as well. The styloid and chordal eminences are inferior and posterior to the pyramidal eminence, respectively. The posterior tympanum is derived virtually in its entirety from the second branchial arch.7,12 These recesses may be hidden from view during surgery and are often the site of residual collections of granulation tissue or CH. They are consistently well seen on axial CT section.4,49,50 CT visualization of the sinus tympani is especially important preoperatively, as extensive involvement in this location may require a retrofacial (nerve) surgical approach (Fig. 3.14 and Fig. 3.24). A highly positioned jugular bulb and a contracted space between the facial nerve and the posterior semicircular canal preclude this type of exploration.47,48
These structures form the posterior portion of the tympanic cavity proper. The lateral border is the TM, and the medial border is the labyrinth, particularly the promontory. The roof of the epitympanum, which is referred to as the tegmen tympani, separates the epitympanum from the middle cranial fossa. The inferior wall of the tympanic cavity (hypotympanum) is separated by plates of bone anteriorly from the carotid canal and posteriorly from the jugular bulb (Fig. 3.22).
Blood Vessels and Nerves
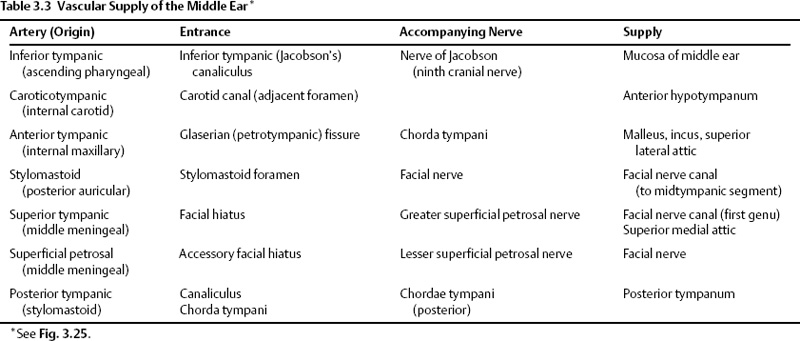
Numerous arteries contribute to the vascular supply of the middle ear (Table 3.3 and Fig. 3.25).11,51 The inferior tympanic artery is most often a branch of the ascending pharyngeal artery. It enters the middle ear via the inferior tympanic (Jacobson’s) canaliculus with a similarly named branch of CN IX. It anastomoses with the tiny caroticotympanic branch (internal carotid artery [ICA]) and provides the major supply of the hypotympanum. The anterior tympanic artery most often arises from the internal maxillary artery and subsequently courses posteriorly through the petrotympanic (glaserian) fissure with the chorda tympani nerve into the middle ear.11 This vessel provides the primary blood supply of the malleus and incus and the superior and lateral walls of the attic.
The vascular supply of the distal ossicular chain and facial nerve canal is derived from the stylomastoid, superior tympanic, and superficial petrosal arteries. The stylomastoid artery arises either from the posterior auricular or occipital branches of the external carotid artery and enters the stylomastoid foramen. It contains a posterior tympanic branch, which courses with the chorda tympani nerve via the canaliculus chorda tympani into the middle ear.51 The superior tympanic and superficial petrosal arteries are the first two endocranial branches of the middle meningeal artery just after its entrance into the cranial cavity via the foramen spinosum. These two branches course with the greater and lesser superficial petrosal nerves, respectively, into the middle ear.
The nerve supply of the middle ear mucosa is primarily via the tympanic plexus of CN IX.
Pneumatization
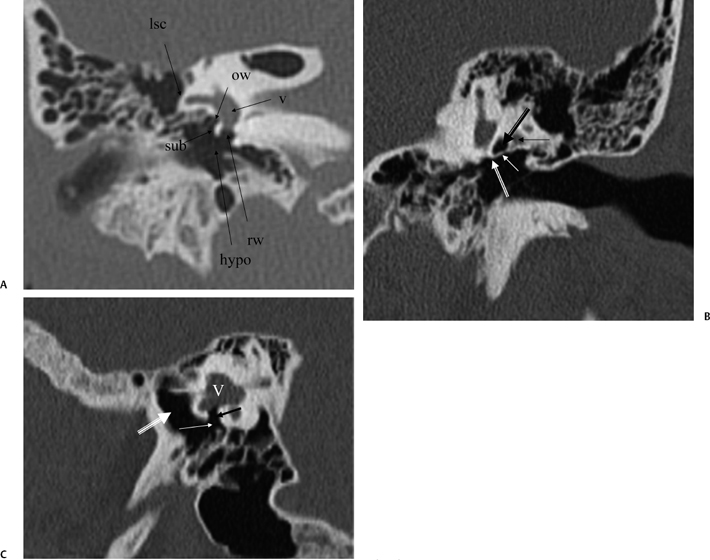
Fig. 3.23 (A) Coronal anatomy (lsc, lateral semicircular canal; ow, oval window [posteriormost aspect]; v, vestibule; rw, round window; hypo, hypotympanum; sub, subiculum). (B) Coronal computed tomography image. Outlined black arrow, oval window (posterior portion); thin white arrow, ponticulus; outlined white arrow, round window (cephalad border); thin black arrow, facial nerve canal, second genu. (C) Vestibule (V). Black arrow, round window (leading to scala tympani of basilar cochlear turn); white arrow, round window niche; large white arrow, hypotympanum.
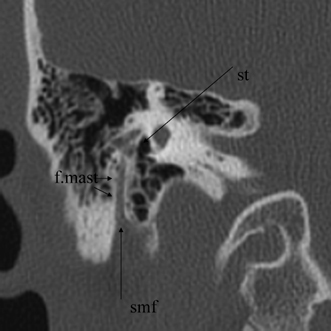
Fig. 3.24 Coronal anatomy (st, sinus tympani; f.mast, facial nerve, mastoid segment; smf, stylomastoid foramen).
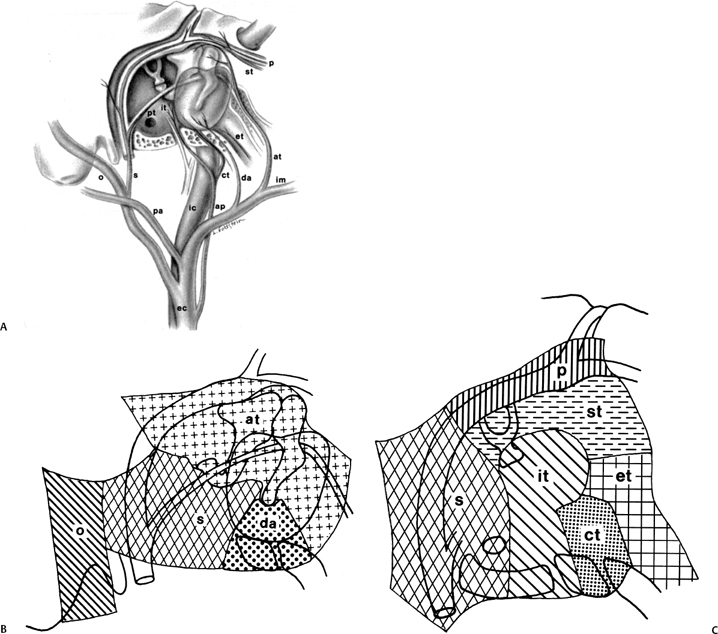
Fig. 3.25 (A) Vascular supply of the middle ear, sagittal (oblique) drawing. (B,C) Insets (ec, external carotid; ic, internal carotid; pa, posterior auricular; ap, ascending pharyngeal; o, occipital; im, internal maxillary; da, deep auricular; at, anterior tympanic; s, stylomastoid; it, inferior tympanic; st, superior tympanic; ct, caroticotympanic; pt, posterior tympanic; p, petrosal; et, eustachian tube). (Adapted from Hesselink JR, David KR, Taveras JM. Selective arteriography of glomus tympanicum and jugulare tumors: techniques, normal and pathologic arterial anatomy. AJNR Am J Neuroradiol 1981; 2:289–297. Reprinted with permission.)
The degree of pneumatization of the temporal bone is variable and dependent on several factors, including nutrition, heredity, and environment.7,24,35 The hereditary theory suggests that mastoid size is independent of the status of the mesotympanum. The environmental theory suggests that the degree of childhood middle ear disease determines the size of the mastoid air cell system.52 There is no uniform agreement on the embryologic onset, which is typically a symmetric process unless complicated by inflammatory disease. The frequency of bacterial infection (eustachian tube function) therefore plays a critical role. The eustachian tube is responsible for middle ear ventilation, protects it from pathogenic organisms, equilibrates pressure across the TM, and allows drainage of secretions. It is essential for maintenance of normal ventilation.1
Individuals with unilateral depressed pneumatization will often have a history of chronic otitis media (COM). Interestingly, patients with cystic fibrosis typically have excellent pneumatization and a low incidence of infection.53
Pneumatization is described as pneumatic when complete, diploic when partial, and sclerotic when essentially absent.54 In the latter two categories aeration is limited to an antrum and central mastoid tract of variable size. The nonpneumatized portion consists primarily of bone marrow (diploic) or dense bone (sclerotic).24 Five pneumatized regions are described: the middle ear, mastoid, perilabyrinthine, petrous apex, and accessory regions.55 There is extensive intercommunication (Table 3.4).
|
Source: From Schuknecht HF. Pathology of the ear. 2nd ed. Philadelphia: Lea & Febiger; 1993. Reprinted with permission.
The middle ear is divided into three compartments in the coronal plane (epitympanum, mesotympanum, hypotympanum) and in the axial plane (protympanum, mesotympanum, posterior tympanum) (Fig. 3.26). The mesotympanum is located immediately opposite the pars tensa and may be separated from the more superiorly placed epitympanum and more inferiorly placed hypotympanum by tangential lines drawn through the superior and inferior margins of the EAC, respectively. Similarly, the mesotympanum may also be separated from the more anteriorly located protympanum and more posteriorly located posterior tympanum by tangential lines drawn through the anterior and posterior aspects of the EAC, respectively (Fig. 3.26). Thus, there are five definable areas of pneumatization within the middle ear.7
The epitympanum (attic) contains the malleus head and incus body and lies within the notch of Rivinus, a fan-shaped recess in the tympanic bone. The tympanosquamous suture line forms the anterior boundary and the tympanomastoid suture, its posterior boundary.
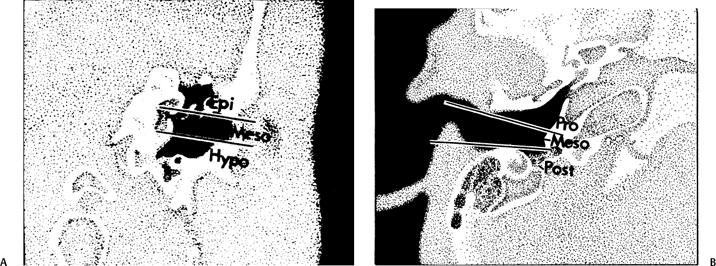
Fig. 3.26 Artist’s rendering of compartments of middle ear. (A) Coronal (Epi, epitympanum; Meso, mesotympanum; Hypo, hypotympanum). (B) Axial (Pro, protympanum; Meso, mesotympanum; Post, posterior tympanum). (Adapted from Hesselink JR, David KR, Taveras JM. Selective arteriography of glomus tympanicum and jugulare tumors: techniques, normal and pathologic arterial anatomy. AJNR Am J Neuroradiol 1981;2:289–297. Reprinted with permission.)
Two communications have been extensively described between the mesotympanum and epitympanum. These are referred to as the anterior and posterior tympanic isthmus.7,44,49,56,57 These isthmi are vertical in orientation and pierce the tympanic diaphragm, a series of mucosal folds and ligaments that separate the mesotympanum from the epitympanum. The anterior isthmus lies between the tensor tympani tendon and the incudostapedial region, and the posterior tympanic isthmus between the short process of the incus and the stapedius tendon. They may be appreciated in cross section on axial CT.4,5,49 They are prone to occlusion in the poorly pneumatized middle ear. Recently, other authors have disputed the anatomic presence of these isthmi and doubted that occlusive change predisposes to CH development.58,59
The mastoid region is subdivided into the mastoid antrum, which communicates with the epitympanum (attic) via the aditus, the central mastoid tract (direct inferior extension of the antrum), and the peripheral mastoid area (additional cells that arise from the antrum).37
This latter region is further subdivided into tegmental cells (above the EAC), posterosuperior sinodural cells, posteroinferior sinal cells (closely related to the sigmoid sinus), facial cells (related to mastoid segment of facial nerve canal), and mastoid tip cells, which are divided into medial and lateral portions by the digastric groove.7,24 The mastoid antrum is present at birth. Peripheral pneumatization continues through early childhood.
The tegmen is bone of variable thickness that forms the roof of the epitympanum/middle ear (tegmen tympani) and the roof of the mastoid (tegmen mastoideum) (Fig. 3.8). It is lined on the superior surface by dura and on the undersurface by mucosa.60 As such, the tegmen provides a crucial barrier preventing the spread of infection and leakage of cerebrospinal fluid. In autopsy series, 1 to 5 focal defects are described in the tegmen in 15 to 34% of cases, most often in the well-pneumatized mastoid. Rarely, multiple defects are described and may be the source of some morbidity including spontaneous CSF leaks and pneumocephalus.
The anterolateral portion of the mastoid is derived from the squamous portion of the temporal bone; the posteromedial portion including the mastoid tip arises from the petrous portion.61 The Koerner’s (petrosquamous) septum (KS) is a structure of interest to both the radiologist and otologic surgeon due to the surgical implications when it is particularly thick, as it may be confused with the medial wall of the antrum (see Normal Variations subsection below) (Fig. 3.19). This structure begins at the glenoid fossa and extends inferiorly lateral to the facial nerve canal toward the mastoid tip. The KS is variably sized and predisposes the patient with chronic otitis to so-called attic block.46 This septum may be divided into three portions: ventral (temporomandibular), middle (tympanic), and dorsal (mastoid). The ventral extremity can be used as a surgical landmark for the mastoid segment of the facial nerve canal.62 The septum appears essentially absent in the well-pneumatized temporal bone; however, recent studies indicate that its size bears no relationship to the extent of pneumatization itself.62,63
The perilabyrinthine cells are located posterior to a vertical plane passing through the modiolus of the cochlea and are described as being either supralabyrinthine or infralabyrinthine.7 The cochlea and the petrous apex region are anterior to this plane and consist of peritubal cells (adjacent to the eustachian tube, antero-lateral to the carotid canal) and apical cells (anteromedial to the carotid canal).55,64 Pneumatization is far more common in the peritubal region (65%). These peritubal cells often communicate directly with the eustachian tube instead of the tympanic cavity. This anatomic fact may explain persistent CSF leaks in individuals who have undergone middle ear obliterative surgery.65 Eradication of CSF otorhinorrhea in this circumstance requires obliteration of several millimeters of the osseous eustachian tube orifice. This may be difficult due to the anatomic proximity of the carotid canal.
Accessory pneumatization may extend beyond the confines of the aforementioned regions into the zygomatic, squamous temporal bone, occipital bone, and styloid process.24,37
Developmental tracts of pneumatization may serve as pathways for disease within the temporal bone. These include the posterosuperior cell tract, the posteromedial cell tract (superior retrolabyrinthine), the subarcuate cell tract (translabyrinthine), the perilabyrinthine tract, and the peritubal tract. These tracts all intercommunicate and are named according to location. Of interest is that the petrous apex may be pneumatized by all of these tracts.
The petrous apex is a truncated pyramid medial to the inner ear, the carotid artery, and the eustachian tube, and is pneumatized in 30 to 35% of temporal bones.66–70 This
pneumatization occasionally consists only of a single giant air cell.71 Although these giant air cells were perhaps a differential diagnostic problem with conventional radiographs and multidirectional tomography, their characteristic air density on CT presents little diagnostic difficulty. Interestingly, it is the nonpneumatized petrous apex that can cause difficulty on magnetic resonance imaging (MRI) (Fig. 3.27).67 Bright signal from marrow fat can cause confusion because pathologic processes associated with hemorrhage, specifically cholesterol granuloma, may be imitated by the rapid T1 relaxation times. Computed tomography will typically be diagnostic in identifying asymptomatic petrous apex penumatization. T2-weighted sequences should reveal less signal intensity and as such will easily document the presence of bone marrow (fat). The os suprapetrosum of Meckel is a small bony structure located adjacent to the petrous apex that occurs as a normal variation.72
A mastoid pneumocele is a thin-walled generalized air cell enlargement presumably due to a one-way ball valve. This is differentiated from a pneumatocele, which is a collection of loculated air under tension within the soft tissues outside the abnormal cell. Compromise of the external auditory canal associated with conductive hearing loss has been reported in this context.73 Defects within the tympanosquamous and tympanomastoid sutures are likely causative. Pneumocephalus has also been described.
Normal Variations
A congenital gap in the bony facial nerve canal is referred to as a dehiscence.74,75 Such dehiscences are a well-known surgical hazard and occur most commonly in the midtympanic segment above the oval window niche.76 A dehiscence is difficult to appreciate with CT, as the bone is quite thin, but recently observers have had some success in this regard.74 The facial nerve is seen in cross section beneath the lateral semicircular canal on coronal images at the level of the vestibule. The surgeon should be cautioned if there is an especially noticeable inferior facial nerve convexity in this plane, particularly when the oval window niche appears compromised, because this may represent protrusion of the nerve through the dehiscence (Fig. 3.28). Such a protrusion will directly result in conductive hearing deficit (CHD) on rare occasions. The status of this segment of the nerve should always be addressed when surgery for otosclerosis is being contemplated as it represents a potential hazard. In individuals with extensive middle ear debris filling the middle ear cavity, the diagnosis may not be possible because the density of CH and granulation tissue is identical to that of the nerve. It is worthwhile to search for a “notch” on the inferior surface of the lateral semicircular canal in these patients. In my experience, a clinically significant dehiscense/protrusion is usually not present when this notch is visualized. An especially pronounced protrusion of the nerve into the oval window niche could conceivably masquerade as a schwannoma or a congenital CH.
Preoperative knowledge of several other anatomic variations may be very helpful to the otologic surgeon.4,77 The dehiscent or high-riding jugular bulb is discussed in Chapter 4.27 On occasion, we will identify a sigmoid sinus that is much more anteriorly and laterally located than normal (Fig. 3.29). This is much more common on the right presumably because the superior sagittal sinus often drains preferentially into the right transverse sinus.78 The size of the mastoid antrum may be compromised, and this variation could make surgery more complicated, depending on the approach preferred by the surgeon.
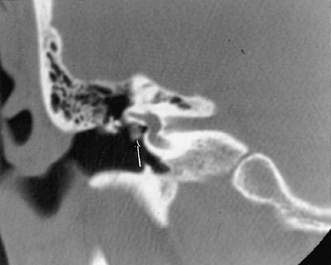
Fig. 3.28 Dehiscent (protruding) facial nerve. Coronal computed tomography image, right ear. There is a prominent inferior convexity to the tympanic segment of the facial nerve (arrow). Such a finding should always be called to the surgeon’s attention.
Specifically, a postauricular approach could be quite hazardous in the presence of this type of sigmoid sinus anomaly. The distance between the sinus and the EAC varies directly with the degree of pneumatization of the mastoid.79
On occasion, we will encounter an extremely deep sinus tympani (Fig. 3.30).4 Surgical exoneration of associated debris within this recess might then be difficult, and the surgeon should be cautioned. The difficulties that may be encountered by the surgeon in the tiny middle ear cavity are self-explanatory.47 A thick Koerner’s septum has been described in the past to be a source of surgical concern. In this situation the surgeon may believe that the entire antrum has been explored, when indeed the more medial aspect has not.60,61,77 The low-lying middle cranial fossa dura represents an obvious surgical hazard (Fig. 3.31). This occurs due to an absence of tegmental pneumatization (above the EAC) in those with congenitally thin superior EAC margins. This phenomenon is further discussed in Chapter 2. Other variations and anomalies will be considered under their appropriate sections and subsections.
Embryology
In this section, I provide a brief overview of the embryological development of the middle ear. For more detail, the reader is referred to Anson and Donaldson.33
The eustachian tube and tympanic cavity are formed from the first pharyngeal pouch (endoderm of tubotympanic recess), which is a foregut outpocketing.30,80,81
The dorsal end of the pouch develops initially into the eustachian tube and subsequently forms tympanic cavity.12 The extensions of the tympanic cavity, the attic (epitympanum), antrum, and mastoid air cells form after the tympanic cavity is developed. As these structures develop, the mesenchyme is replaced by endodermal epithelium. The tympanic cavity reaches adult size by 37 weeks of gestation.
Four endothelial primary sacs develop from the first pharyngeal pouch between the 10th and 30th weeks of gestation and form the tympanic cavity. These include the saccus anticus, saccus posticus, saccus superior (squamous), and saccus medius.12 Mucosal folds form where these sacs contact each other. The saccus medius is particularly important because it is responsible for the development of most of the epitympanum, antrum, and mastoid air cell system.
The saccus medius consists of three smaller saccules, the most medial of which forms Prussak’s space. The anterior epitympanum may be formed by the saccus anticus in some circumstances. When this occurs, the anterior and posterior epitympanum do not communicate.45 The saccus superior lies between the malleus handle and incus long process and is responsible for pneumatization of the squamous temporal bone. Areas pneumatized by the saccus medius and saccus superior subsequently become separated by a variable petrosquamous lamina (Koerner’s septum). The saccus posticus forms the recesses and ridges of the posterior mesotympanum.
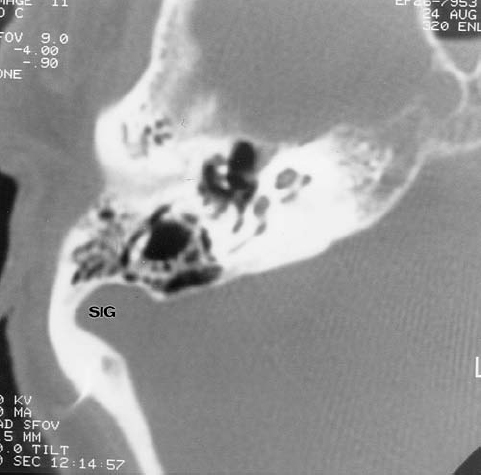
Fig. 3.29 Normal variant. Anteriorly and laterally placed sigmoid sinus (SIG).
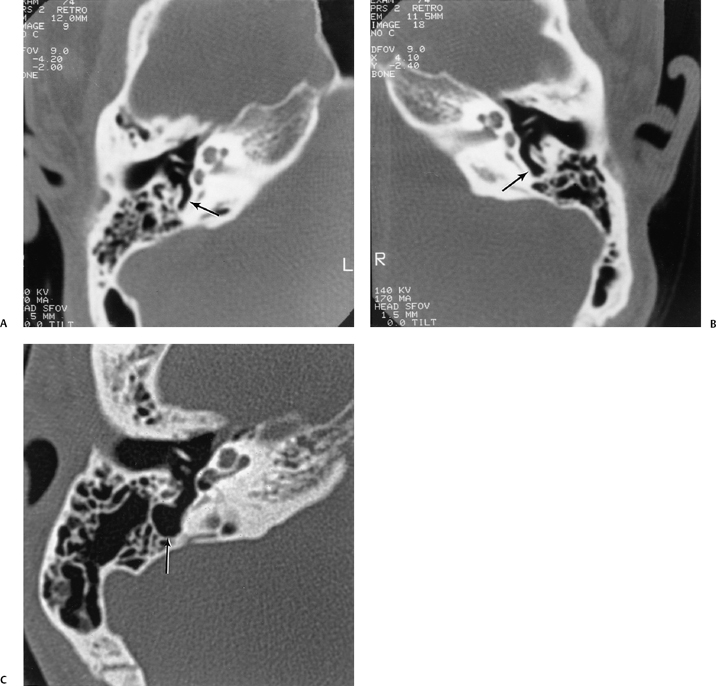
Fig. 3.30 Usually deep sinus tympani (arrow). This represents a site where cholesteatomatous debris may be out of the direct vision of the operating surgeon. (A) Right ear. (B) Left ear. (C) Different patient, sinus tympani of extraordinary size. (C, Courtesy of Curtis Wushensky, MD.)
Pneumatization of the tympanic cavity and epitympanum is complete by week 34 of gestation in most cases; however, further pneumatization of the temporal bone and the rest of the mastoid air cell system continues for a variable time into childhood.7
The first and second branchial arches (mesoderm) differentiate into the ossicular chain and its supporting ligaments, muscles, and tendons (Fig. 3.13F). The first branchial arch (Meckel’s cartilage) develops into the head of the malleus, the tensor tympani muscle and tendon, and the body and short process of the incus.82 The second branchial arch (Reichert’s cartilage) develops into most of the rest of the ossicular chain and also into the stapedius muscle and tendon. Other structures arising in whole, or in part, from the second arch include the mandibular condyle, styloid process, and facial nerve canal.83 Ossicular development occurs simultaneously with the formation and differentiation of the middle ear cavity and its outpouchings. The second half of this interval is primarily concerned with ossification, the ossicles having achieved adult size by the 15th week.84 Formation of the stapes is not complete until week 38.85 Early in gestation the stapes primordium is pierced by the stapedial artery and is separated from the developing facial nerve and pyramidal eminence (laterohyale) by the interohyale, which becomes the stapedius tendon.86 The stapes foot-plate has two layers: the tympanic portion, which is derived from the second brachial arch, and the vestibular portion (with its annular ligament), which develops from the otic capsule.81,84,85 The ossicles change little during life and, similar to the otic capsule, demonstrate a limited capacity for repair.
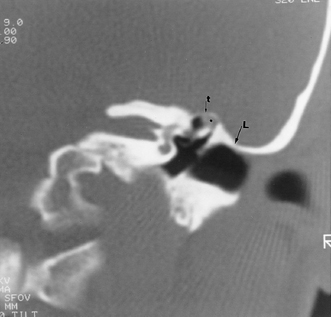
Fig. 3.31 Low middle cranial fossa dura. Coronal computed tomography image. The tegmental air cells above the external auditory canal are absent, and the temporal lobe is in direct apposition to the superior margin of the external auditory canal (L, arrow). Note that this patient does have a tiny cholesteatoma (*) within the attic. The tegmen tympani (t, arrow) is intact. The surgeon should be aware of this configuration prior to surgery.
The TM and the supportive tympanic ring are formed by the 18th week of gestation. Portions of the TM are derived from all three germ layers. The outer epithelial layer is derived from the ectoderm of the first branchial groove (external auditory meatus). The middle fibrous layer is derived from the mesoderm, which insinuates itself between the tympanic cavity and the first branchial groove. The inner mucosal layer is derived from the endoderm of the first pharyngeal pouch.30
The tympanic ring (tympanic bone) is formed in membranous bone from four ossification centers. It is virtually completely developed by the 15th week of gestation. There is a defect in the ring superiorly, which is known as the notch of Rivinus. The TM inserts in this location. The tympanic ring provides the scaffolding for the TM. The TM is relatively horizontal at birth and does not assume the adult vertical orientation until 3 years of age.81 The tympanic ring also contributes to the development of the styloid process. It is this tympanic bone that forms the sides and floor of the bony EAC as it elongates.
Pathology and Treatment
Inflammatory Disease
Despite the obvious interrelationship, acute otomastoiditis (AOM) and chronic otomastoiditis (COM) are considered as two different disease processes for the purposes of this communication. Acute otomastoiditis is usually caused by bacterial infection often superimposed on eustachian tube obstruction, and chronic otomastoiditis by longstanding eustachian tube dysfunction.
Similarly, each disease has its own specific set of complications. With respect to AOM, complications include middle ear effusion, coalescence, subperiosteal abscess, labyrinthitis, and petrous apicitis, as well as intracranial involvement such as meningitis, abscess formation, and dural sinus occlusive disease (see Table 3.5). COM may result in middle ear effusion, TM retractions, acquired CH, granulation tissue, cholesterol granuloma, ossicular fixation, and ossicular erosion. Certainly, complications of AOM may be superimposed on COM. Facial nerve dysfunction may occur as a result of acute or chronic disease. Despite this common misconception, there is no evidence that acute or chronic otitismedia are more prevalent in human immunodeficiency virus (HIV) patients.87 Aggressive fungal or pseudomonas mastoiditis may be seen, however, in severe HIV cases.88,89
|
As indicated in the previous paragraphs, middle ear effusions occur with both AOM and COM. Children are predisposed to AOM and effusion for several reasons. The eustachian tube in children is inherently dysfunctional due to its relatively horizontal orientation and shorter length. Obstruction of the eustachian tube by prominent adenoidal tissue, release of inflammatory mediators from adenoidal mast cells and adenoidal tissue acting as a reservoir for bacteria are all mechanisms for the development of middle ear effusion.90
The reader is cautioned that identification of debris suggestive of inflammatory disease within the mastoid is common, especially in children.91 Perhaps 10 to 15% of children and a substantial percentage of adults examined with MRI for other reasons will have heterogeneous T2-weighted hypersignal within the peripheral mastoid and middle ear proper despite the absence of a history of otitis media. The reason for this is unclear, but the observer should not misconstrue this common finding to be necessarily indicative of clinically significant inflammatory disease. A common error is to refer to abnormal signal in this region as mastoiditis, a clinical diagnosis. I refer to this type of finding as “nonspecific mastoid debris” and recommend CT, if clinically indicated.
Acute Otomastoiditis and Complications
Most cases of acute otomastoiditis occur in children and manifest clinically as otalgia, fever, and erythema/edema of the TM. The middle ear and mastoid are generally considered an extension of the upper respiratory tract and subject to bacterial invasion via the eustachian tube. Mucoperiosteal inflammation results initially in serous effusion, which may become mucoid or purulent.92 Fluid levels are commonly demonstrated at CT provided that there are two orthogonal planes (axial and coronal). Previously, we referred to the aditus ad antrum, an inherently narrow communication between the epitympanum (attic) and the mastoid antrum. Swollen mucosa may block the aditus, which traps secretions in the peripheral mastoid with subsequent development of AOM. Effective therapy is crucial at this juncture.
AOM is most often caused by bacterial infection. Streptococcus pneumoniae (pneumococcus) and Haemophilus influenzae account for 65 to 80% of cases.93 The latter agent is less common but more aggressive and associated with a higher incidence of meningitis.88 Proteus and Pseudomonas species are less common culprits.94 The development of antibiotics, of course, resulted in a remarkable decline in the incidence of the complications of this disorder. Recently, however, there has been somewhat of an upsurge due to drug-resistant organisms (penicillinase-producing Streptococcus pneumoniae and beta-lactamase-producing strains of Moraxella and Haemophilus) and a change in microbial flora.95
Mycotic disease is unusual. These infections may be invasive when they occur in the immunocompromised host.96 Type I infections are limited to the EAC (otitis externa). Type II infections involve extension into the mastoid cavity (mastoiditis). Type III is invasive mastoiditis with facial palsy, and type IV infection is a fulminant skull base osteomyelitis.97–99
Tuberculous otomastoiditis (TOM) is increasing in prevalence due to the rising incidence of immunocompromised hosts. There are many possible methods of inoculation, but most commonly it is either hematogenous or extends directly from the nasopharynx. Classically, these patients present with chronic painless otorrhea and an intact TM; however, wide variations in presentation have been recently described.100,101 Pain, purulence, and EAC/TM involvement are all now considered to be common presentations. Ossicular erosion, aggressive tumor-like middle ear/mastoid destruction, and lymphadenopathy are all associated with this disease (Fig. 3.32). The lymphadenopathy commonly involves the postauricular region but may involve the parotid gland or other upper cervical lymph nodes.100 TOM should be considered in any patient regardless of immune state who fails to respond to antibacterial therapy.102,103 Peridural disease and facial nerve involvement are especially common in these patients. In fact, the classic clinical triad of TOM is multiple TM perforations, “pale” granulation tissue, and facial paralysis.104 Atypical mycobacteria are most frequently associated with chronic intractable granulation tissue. Often, these patients are also immunosuppressed.105
Complications of acute otomastoiditis include coalescent mastoiditis, subperiosteal abscess, Bezold abscess, meningitis, parenchymal/extracerebral abscess, empyema, dural sinus occlusive disease, otitic intracranial hypertension (otitic hydrocephalus), facial nerve involvement, labyrinthitis, and petrous apicitis. Occasionally, these complications may occur superimposed upon chronic otitis media. Such superinfection is especially dangerous when CH is present. In these cases, careful study of the tegmen tympani and sigmoid sinus plate is required for reasons that will be described in subsequent sections of this chapter. When this type of bony defect is present, evaluation with MRI (and MR angiography [MRA]) is strongly recommended.106,107
Coalescent Mastoiditis
Fortunately, the vast majority of patients with acute otomastoiditis are cured after a course of antibiotics, and no imaging procedures are necessary.11 Should CT or MRI be performed at this time, nonspecific debris would be identified, typically associated with several fluid levels (Fig. 3.33). CT will demonstrate the integrity of the mastoid septa, ossicular chain, and the internal and external mastoid cortex.108 The turning point for these patients is when mucoperiosteal disease becomes bone disease with enzymatic resorption of mastoid septa and the development of an intramastoid empyema. This is referred to as coalescent mastoiditis.109,110 Osteoclastic dissolution of the pneumatic cell walls is likely a result of hyperemia, local acidosis, and subsequent calcium dissolution.84,92 The diagnosis of coalescent mastoiditis often requires the demonstration of subtle bony changes, which must be carefully sought in all AOM patients; therefore, high-resolution CT is the best imaging modality available during this interval (Fig. 3.34, Fig. 3.35, and Fig. 3.36). Subtle evidence of alternations of these mastoid septations may be clinically significant and reflect antibiotic failure. Often a comparison to the opposite side is needed in this regard (despite the fact that mastoid pneumatization is not always symmetric). Fortunately, coalescence is usually not subtle, and the diagnosis will be obvious. Importantly, when coalescent disease is the result of aditus obstruction or perhaps attic blockade (isthmus obstruction), the TM and middle ear may appear normal otoscopically. This is referred to as latent mastoiditis due to the paucity of clinical clues.92
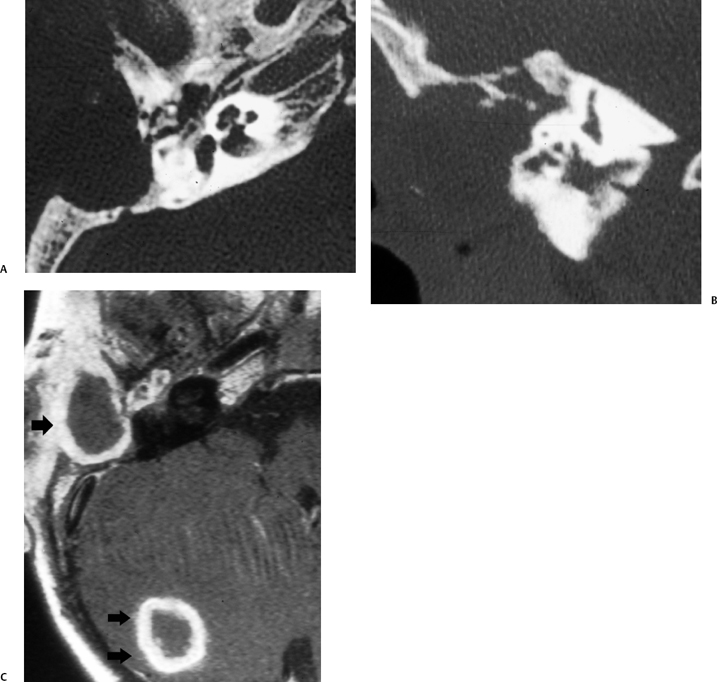
Fig. 3.32 Tuberculous otomastoiditis with parenchymal abscess. (A) Axial and (B) coronal computed tomography images reveal a destructive process sparing the inner ear. (C) Contrast-enhanced T1-weighted magnetic resonance image reveals a thick rind of enhancement surrounding the mastoid debris (single arrow) as well as a mature abscess in the periphery of the cerebellar hemisphere (double arrows).
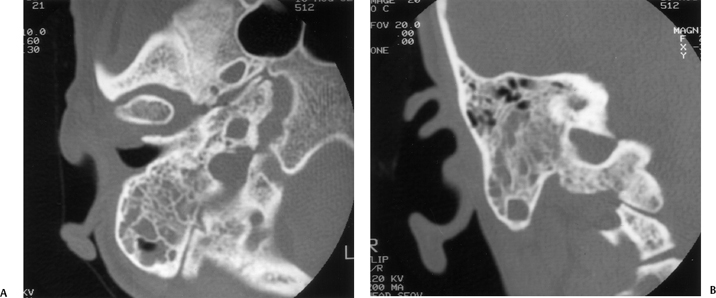
Fig. 3.33 Uncomplicated acute otomastoiditis. (A) Axial and (B) coronal computed tomography images. There is diffuse debris throughout the mastoid. Note the preservation of the integrity of the mastoid septations as well as the internal and external mastoid cortices.
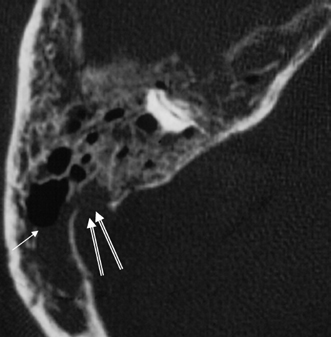
Fig. 3.34 Acute coalescent mastoiditis. Magnified axial computed tomography image reveals diffuse mastoid debris with fluid level (arrow). All of the septations are thin and irregular. There is a sigmoid sinus plate defect (outlined arrows), which must be viewed with suspicion.
Subperiosteal Abscess
In addition to evaluating the status of the mastoid septa, the internal mastoid cortex and external mastoid cortex must be carefully examined. A subperiosteal abscess typically develops via direct extension of the inflammatory debris through a defect in the external mastoid cortex. Such collections are often palpable (Fig. 3.37 and Fig. 3.38). They are usually postauricular due to the thin trabecular bone in this region (Macewen’s triangle).11 Abscess formation should not be confused with the edema, which occurs in this location secondary to thrombosis of mastoid emissary veins (Griesinger’s sign). Preauricular abscess formation is possible if the infection preferentially spreads along the zygomatic root. Even more rare is the Luc’s abscess which develops deep to the temporalis muscle.
Bezold Abscess
The Bezold abscess is analogous to the superiosteal abscess occurring when the bony defect is seen at the mastoid tip (instead of the external mastoid cortex) medial to the insertion of the posterior belly of the digastric (digastric groove) and sternocleidomastoid muscle. This results in inflammatory debris extending inferiorly along the soft tissues of the neck, often with formation of an abscess (Fig. 3.39).111,112,113 Importantly, the inflammatory lesion most commonly lies within the posterior cervical space deep to the sternocleidomastoid muscle, resulting in the absence of a clinically palpable fluctuance.113 Pneumatization of the mastoid tip is a predisposing factor (also analogous to petrous apicitis in this regard, vide infra); therefore, this process is more common in adults than children. For these reasons, temporal bone CT is recommended for unexplained neck abscesses. The potential exists for this abscess to extend inferiorly as far as the mediastinum.
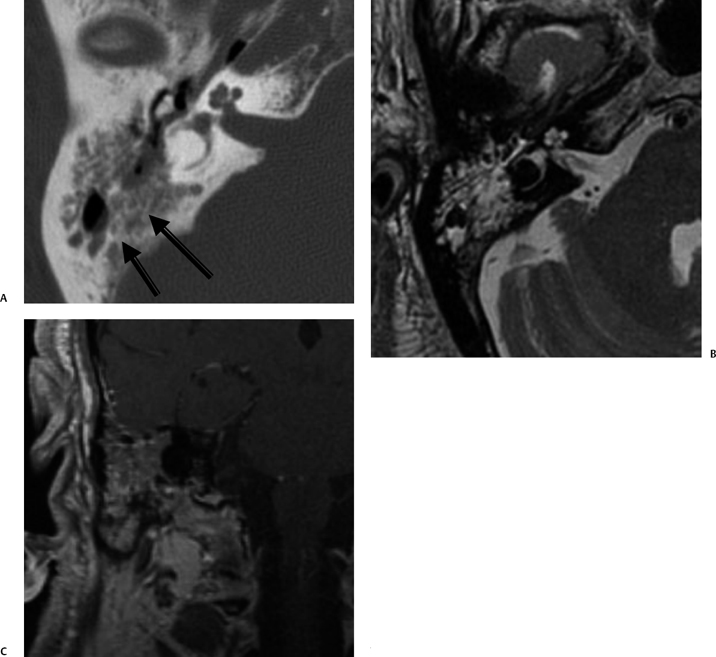
Fig. 3.35 Coalescent mastoiditis. (A) Axial computed tomography image reveals diffuse mastoid debris with extensive coalescent changes in mastoid septations (arrows). (B) Axial T2-weighted MRI confirms debris but not the changes in the bony septations. (C) Coronal-enhanced T1-weighted MRI reveals that debris enhances.
Meningitis, Abscess, and Empyema
Defects in the internal mastoid cortex are of obvious concern, as this leaves the underlying dura adjacent to the sigmoid sinus and cerebellum directly exposed to the inflammatory process. Dangerous intracranial complications such as sigmoid sinus thrombosis, intracerebral or extracerebral (perisinus) abscess formation, and meningitis can occur via direct extension, hematogenous dissemination, or retrograde thrombophlebitis.114 The latter is considered to be the most common mode of spread.
An abscess is a collection of pus lined by a fibrous capsule. When it occurs in the subdural or epidural compartment, it must be distinguished from empyema, which spreads out over a wider area. Individuals with a subdural empyema (SDE) typically have meningitis as well. SDE is a much more likely complication of sinusitis than otomastoiditis.115 Sterile subdural collections (hygroma) are also associated with meningitis in the absence of abscess formation. Rarely, spread of inflammatory disease to the meninges occurs along normal anatomical structures such as the petrosquamous suture and petromastoid canal (subarcuate artery).11 Proteus, Pseudomonas, and Staphylococcus species are often isolated in these advanced cases.116 Abscesses may occur in the middle cranial fossa, but are much more common in the posterior fossa due to osseous destruction in the Trautmann triangle between the sigmoid sinus plate and the sigmoid sinus (Fig. 3.32, Fig. 3.40, and Fig. 3.41).92
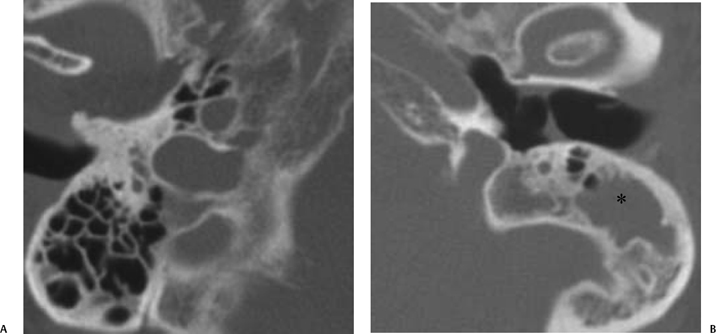
Fig. 3.36 Coalescent mastoiditis, left ear. (A) Magnifed axial computed tomography (CT) image of the normal right ear. Note the normal pneumatization and well-defined septations. (B) Magnified axial CT of the left ear demonstrates debris (*) in the peripheral mastoid in a patient with otalgia and fever. Note the loss of mastoid septations, confirming coalescent disease. The internal and external mastoid cortex is intact.
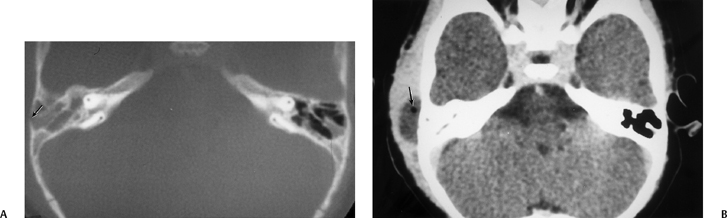
Fig. 3.37 Coalescent mastoiditis, subperiosteal abscess. (A) Axial computed tomography (CT) image. Debris throughout mastoid with thinning and poor definition of mastoid septations (compare with opposite side). Focal defect in external mastoid cortex (arrow). (B) Axial CT image, postcontrast. Low-density mass representing subperiosteal abscess. Note small air bubble (arrow).
Unexplained episodes of meningitis especially in children typically provoke a search for a parameningeal focus, and history/findings compatible with otitis media are carefully sought in this context. Detailed CT investigation of the bony margins of the temporal bone is required. Congenital fistulas, often associated with inner ear malformations, may be present (see Chapter 5). These patients typically have CSF otorhinorrhea. In several published reports, surgical investigation of focal cortical defects along the posterior and middle fossa surfaces of the temporal bone has revealed arachnoid granulations as the cause of recurrent meningitis in a significant number of cases117 (see Chapter 5). Hyrtl’s (tympanomeningeal) fissure normally closes at 24- to 26-week gestation. If persistently patent, this structure may also be responsible for spontaneous CSF otorrhea. These defects are infralabyrinthine adjacent to the round window.118 Spontaneous CSF otorrhea may also be caused by significantly larger defects and be associated with meningoencephalocele. These patients often present with CHD.119
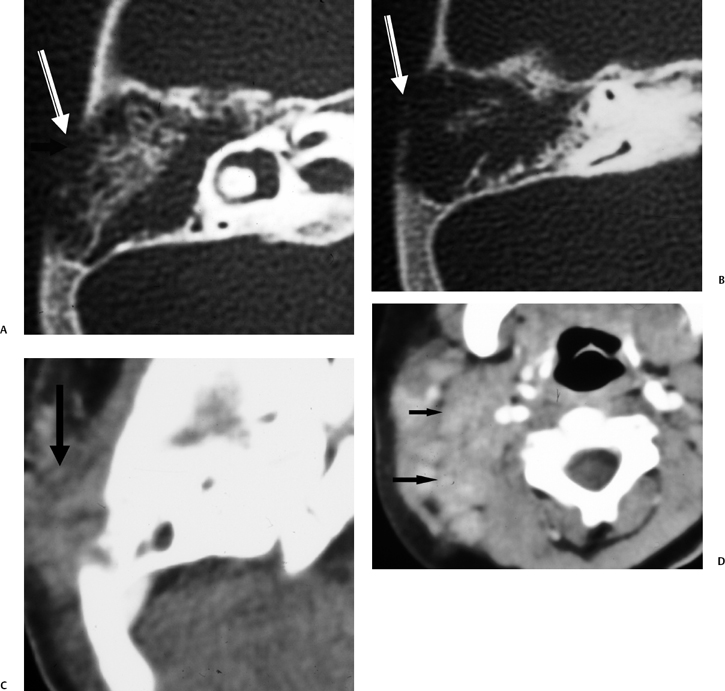
Fig. 3.38 Coalescent mastoiditis with subperiosteal phlegmon and lymphadenopathy. (A,B) Axial computed tomography (CT) images reveal diffusely eroded mastoid septations, indicating coalescent disease, with a large defect in the external mastoid cortex (arrow). (C) Corresponding axial CT image with soft tissue window reveals phlegmonous debris (arrow) rather than subperiosteal abscess, which typically occurs under these circumstances. (D) Axial CT image through neck soft tissue reveals multiple pathologic lymph nodes (arrows).
Meningitis is the most common intracranial complication of acute otomastoiditis.120 Proteus, Pseudomonas, or Staphylococcus species are usually isolated. A brain (parenchymal) abscess usually involves the temporal lobe and presents with symptoms of a mass lesion. Anaerobes such as Bacteroides and Fusobacterium may be the culprit. Interestingly, a brain abscess is often a complication of chronic rather than acute otitis. Epidural and subdural abscess often occurs adjacent to the sigmoid sinus and is strongly associated with bone erosion. Middle fossa extraaxial collections often exhibit somewhat limited extension due to the firm attachment of the dura to the arcuate eminence, a convexity corresponding to the site of the superior semicircular canal.120
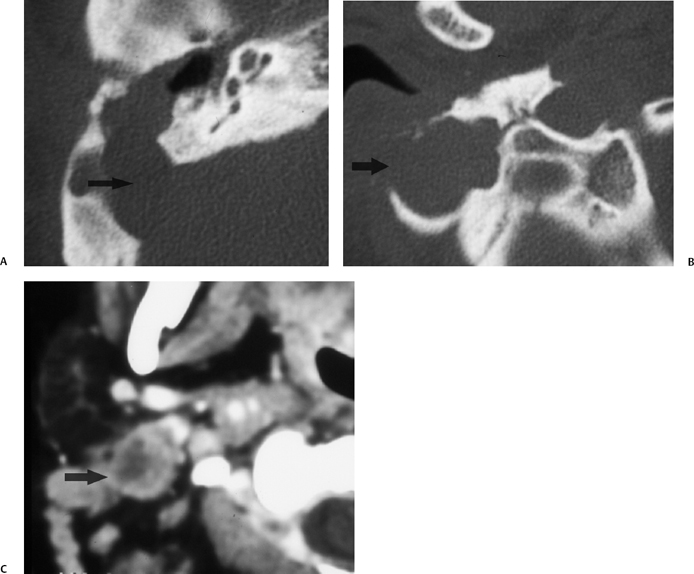
Fig. 3.39 Mastoiditis–Bezold abscess. (A) Axial computed tomography (CT) image reveals diffuse mastoid debris with a large sigmoid sinus plate defect (arrow). (B) More inferior image at the mastoid tip reveals a large area of erosion with a large lateral bony defect (arrow). (C) Axial CT image immediately subjacent to the mastoid tip reveals a classic Bezold abscess (arrow).
Dural Sinus Occlusive Disease
Dural sinus occlusive disease (DSOD) is an extremely dangerous and potentially fatal complication of AOM that may occur via direct extension or result from erosive osteitis and retrograde thrombophlebitis via emissary veins. The majority are associated with epidural abscess. Perisinus inflammation may induce formation of mural thrombus within the sinus lumen secondary to pressure effects, causing the adherence of fibrin and platelets.121,122
This mural thrombus becomes infected and propagates to form an obliterating thrombus.94 Some consider the formation of a thrombus to be a protective mechanism attempting to limit and localize the infection. Severe headaches, high spiking fevers, postauricular edema, sixth nerve palsy, and mental status changes herald the diagnosis clinically. Alterations of posture may be catastrophic for these patients. Antibiotic therapy is the cornerstone of treatment.123 Anticoagulation is controversial. Surgical exploration is often needed. Aspiration of the clot may yield the necessary restoration of flow, but incision into the sinus and removal of the clot may be necessary. Ligation of the sinus is rarely needed, but it has been used to control septic emboli.120
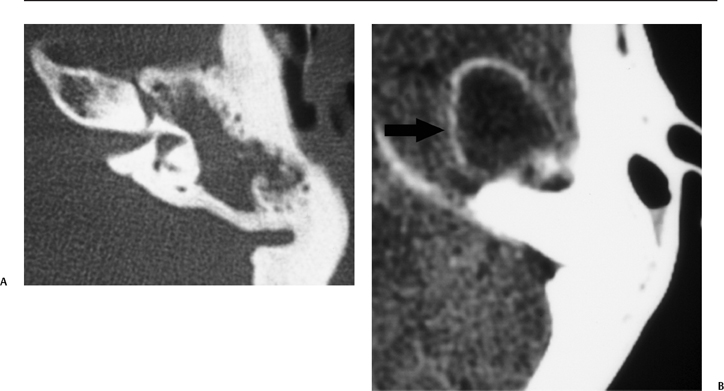
Fig. 3.40 Coalescent mastoiditis with temporal lobe parenchymal abscess. (A) Axial computed tomography (CT) image at bone window reveals diffuse middle ear debris sparing the inner ear. (B) Axial CT image at soft tissue window reveals a well-defined ringenhancing mass in the middle cranial fossa, consistent with abscess (arrow).
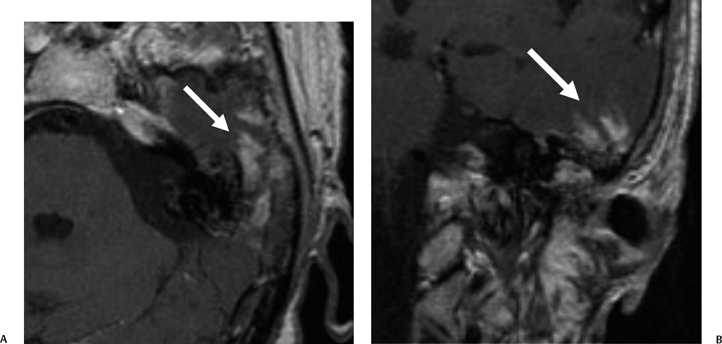
Fig. 3.41 Acute otomastoiditis, cerebritis. (A) Axial and (B) coronal contrast-enhanced T1-weighted magnetic resonance images reveal mastoid debris with a heterogeneous intensely enhancing area (arrow) in the adjacent temporal lobe.
Due to anatomic proximity, involvement of the sigmoid/transverse sinus is most common. In many patients, a clot may propagate antegrade into the internal jugular vein or retrograde to the torcula and superior sagittal sinus.124 Extension along emissary veins to the subcutaneous tissues may also occur. Furthermore, the reader should be aware of the anatomic proximity of the superior and inferior petrosal sinuses, which drain the cavernous sinus. Clot propagation retrograde could thus have further dire clinical consequences. Systemic septic emboli and pulmonary thromboembolic disease also have grave implications.119,125
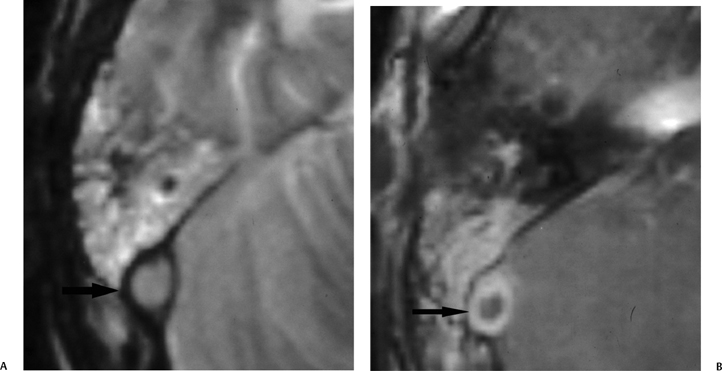
Fig. 3.42 Mastoiditis. sigmoid sinus occlusive disease. (A) Axial contrast-enhanced T2-weighted MRI and (B) T1-weighted MRI reveals enhancing debris throughout mastoid in a patient with spiking fevers. (A) Abnormal hypersignal (arrow) in the expected location of the sigmoid sinus and (B) an area of nonenhancement (arrow) centrally within the sigmoid sinus representing a thrombus.
DSOD remains a difficult imaging diagnosis despite the advent of MRI (Fig. 3.42, Fig. 3.43, and Fig. 3.44). Clinical suspicion is the single most important diagnostic element.126 If examination of conventional spin echo images reveals a flow void in the typical anatomic location of the sigmoid sinus, the diagnosis of occlusive disease is effectively excluded. Similarly, bright signal representing flow-related enhancement (FRE) on gradient echo pulse sequences also lessens the likelihood of this diagnosis. Findings on spin echo images can be particularly perplexing due to the vagaries of flow phenomena. In addition, gadolinium-enhanced T1-weighted images may be confusing as a result of dose-related issues and due to thrombus enhancement, which occurs consistently in chronic cases, presumably secondary to the conversion of the clot into vascularized connective tissue.127 Enhancement with gadolinium also occurs commonly in normal cases due to physiological slow flow.
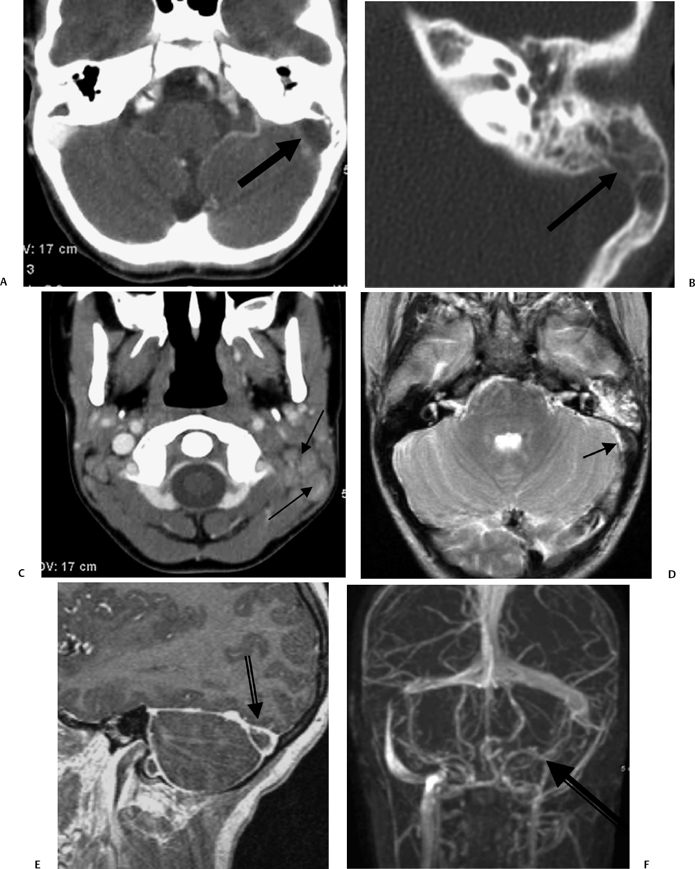
Fig. 3.43 Acute otomastoiditis, sigmoid sinus thrombosis. (A) Axial contrast-enhanced computed tomography (CT) image reveals mastoid debris with a “vacant” nonenhancing sigmoid sinus (arrow). (B) Magnified axial CT, left ear, reveals coalescent disease (arrow) with thinning of the sigmoid sinus plate (arrow). (C) Axial contrast-enhanced CT image reveals lymphadenopathy (arrows), more noticeable on the left. (D) Axial T2-weighted MRI reveals hyperintense mastoid debris with a hypointense area in the vicinity of sigmoid sinus (arrow), representing an acute clot. (E) Sagittal contrast-enhanced T1-weighted MRI reveals a nonenhancing area (arrow), representing thrombosis. (F) Magnetic resonance venography confirms absent flow in the sigmoid sinus and internal jugular vein (arrow). (Courtesy of Deborah Shatzkes, MD.)
When considering this diagnosis, the observer must take into account indirect factors such as absent flow void on spin echo images and absent FRE on gradient echo sequences. These findings are nonspecific, but should spark suspicion under certain clinical circumstances. On occasion, signal characteristics allow for direct visualization of the clot within the lumen of the sinus. This is perhaps most effective in the case of the acute clot (initial stage) on spin echo pulse sequences as the hypointensity elicited by deoxyhemoglobin can ordinarily be distinguished from the signal void produced by rapidly flowing blood. Slightly greater intensity of the clot may be appreciated on the first echo (proton density), and this provides corroborative evidence of the presence of the acute clot. Subacute clot (intermediate stage) is hyperintense on all spin echo pulse sequences and may result in confusion with normal slow flow characteristics; however, subacute clot visualization has been reported in the sagittal sinus, torcula, and sigmoid/transverse sinus complex.124
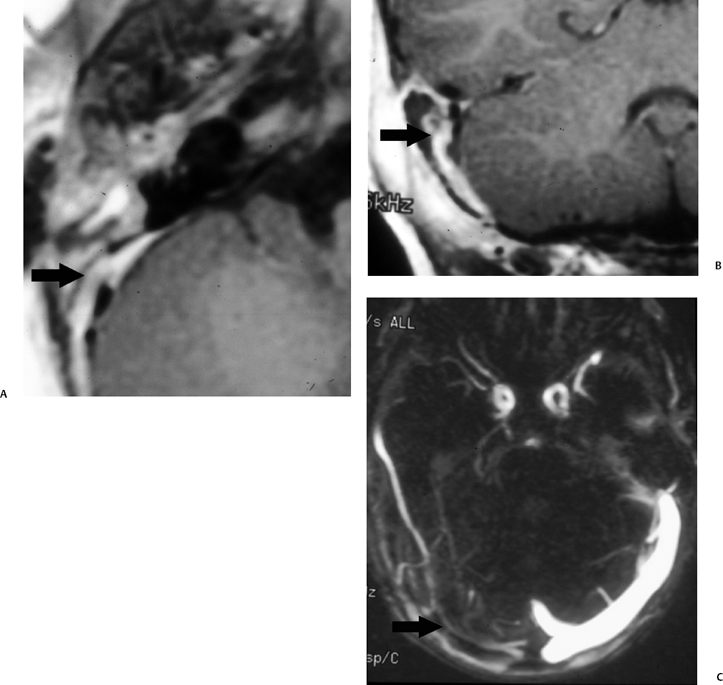
Fig. 3.44 Mastoiditis, sigmoid sinus occlusive disease. (A) Axial contrast-enhanced T1-weighted magnetic resonance image (T1WI) reveals nonspecific enhancing debris throughout the middle ear cleft with absent sigmoid sinus signal void replaced by enhancement (arrow). (B) Coronal contrast-enhanced T1WI reveals confirms abnormal enhancement (arrow) in the region of the expected location of the sigmoid sinus. (C) Magnetic resonance venography reveals absent flow in the transverse (arrow) and sigmoid sinus.
Presently, the combination of pre- and postcontrast MRI and MRA is the state of the art for evaluation of this dangerous clinical condition (Fig. 3.44). Two-dimensional time-of-flight MRA technique is used for screening, but phase contrast technique is often needed, as information regarding the direction of flow may be crucial. The saturation pulse is reversed to gain information about the venous side of the circulation. Recall that dural arteriovenous fistulas (DAVF) are associated with occlusive disease. As such, transosseous vessels (collaterals) and an increased number and size of extracranial vessels should be viewed with suspicion (see Chapter 4).
The observer must be cautious when evaluating MRI/MRA examinations, as there are numerous normal variations. Most important of these is asymmetry, which is extremely common and may be dramatic. This is most often related to the preferential drainage of the superior sagittal sinus into the right transverse sinus. Slow flow is especially common in large veins. Arachnoid granulations may result in focal defects within the walls of the dural sinuses, and clinical correlation is obviously critical.128 CT findings are much less specific. Intense rim enhancement of the sigmoid sinus with lack of internal enhancement is the basis for CT diagnosis (empty delta sign), and this remains a diagnostic mainstay, although its reliability has been questioned.129 In fact, CT may be the only modality used to follow these seriously ill patients. Intraluminal gas bubbles may imply formation of an abscess.125
Otitic Intracranial Hypertension
Historically, the term otitic hydrocephalus (otitic intracranial hypertension [OIH]) has been used to describe the circumstance in which hydrocephalus develops secondary to dural sinus thrombosis presumably due to impaired intracranial venous drainage.11 Actual hydrocephalus is in fact rare. Instead, most use this term to describe increased intracranial pressure occurring secondary to DSOD in the context of complicated AOM. Some reserve this term for a pseudotumor cerebri-like condition unassociated with demonstrable clot.119,124 Others have suggested that OIH is a vasomotor reflex phenomenon originating from the thrombosis rather than mechanical obstruction.
Facial Nerve Involvement
Facial nerve involvement may occur as a complication of AOM or COM (Fig. 3.45).130 The spread of inflammation is facilitated by the common occurrence of developmental dehiscences (perhaps 55%), which have the potential to allow passage of inflammatory by-products. Infections may also spread via the canaliculi, which transmit the neural supply of the chorda tympani and stapedial musculature. The tiny arteries that provide blood supply to the nerve may also be involved. Toxins produced by bacteria may result in facial nerve demyelination.
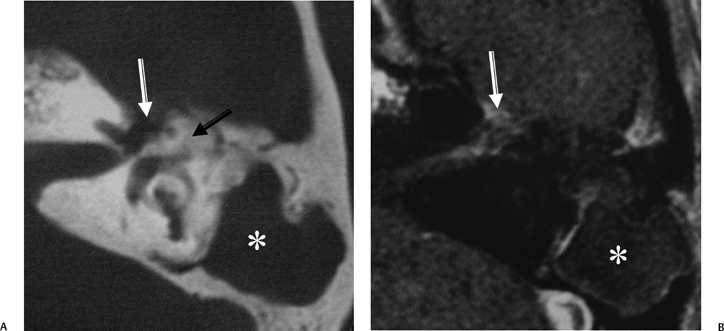
Fig. 3.45 Mastoiditis, acute and chronic, facial nerve involvement. (A) Axial computed tomography image reveals diffuse mastoid disease with coalescence involving the mastoid antrum (*) (no surgery). New bone formation is noted in the attic (arrow). There is erosion near the first genu of the facial nerve canal (white arrow). (B) Corresponding axial contrast-enhanced T1-weighted magnetic resonance image reveals abnormal enhancement of the first genu as well as the intracanalicular segment of the facial nerve (white arrow). (With permission Radiologic Society of North America, 2003.) (See Color Plate Fig. 3.45.)
Labyrinthitis
Labyrinthitis is also a potential complication of acute otomastoiditis. Access to the labyrinth is typically via the round window or oval window (tympanogenic labyrinthitis, see Chapter 5). Exotoxins have also been implicated in the development of labyrinthitis in these patients, suggesting hematogenous dissemination. The classic imaging finding is pathologic contrast enhancement within the normally fluid-filled spaces of the labyrinth on T1-weighted gadolinium-enhanced studies (Fig. 3.46).131 This phenomenon will be considered in much greater detail in Chapter 5. These patients often develop sensorineural hearing loss and vertigo. If the hearing loss is fluctuating, perilymphatic fistula should be considered, especially in children. In a series of 37 children with sensorineural hearing loss secondary to perilymphatic fistula, 76% had documented otitis media.132
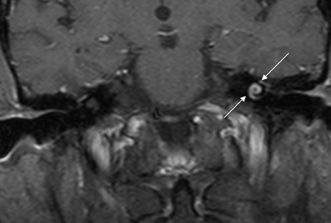
Fig. 3.46 Acute otomastoiditis, labyrinthitis. Coronal contrast-enhanced T1-weighted magnetic resonance image reveals abnormal enhancement of the fluid-filled spaces of the labyrinth (arrows), consistent with labyrinthitis.
Petrous Apicitis
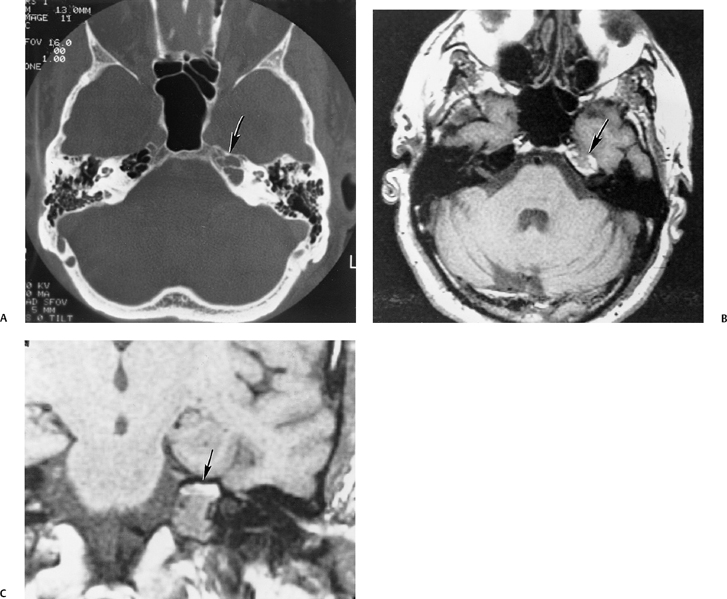
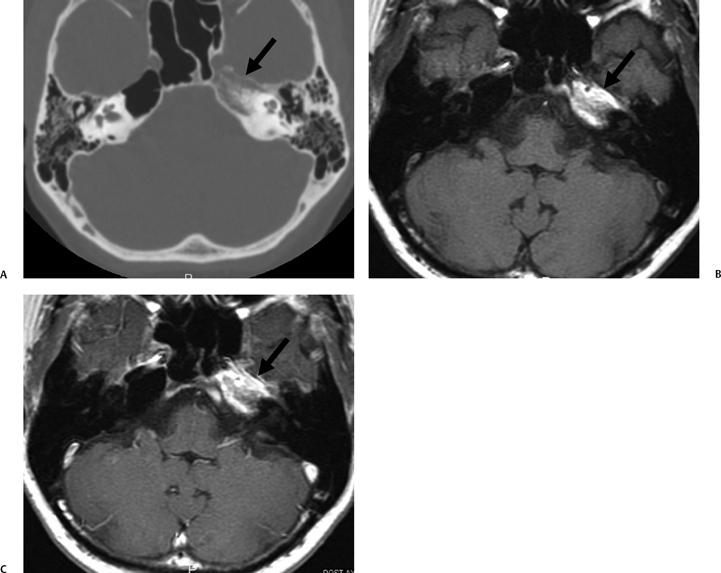
The etiology of petrous apicitis (apical petrositis) has historically been a matter of debate.133 A minority of observers favor an osteomyelitis and consider the petrous apex to be an anatomically distinct and functionally separate portion of the temporal bone. As such, the infection would theoretically spread via retrograde thrombophlebitis along the venous plexus of the petrous ICA. However, the majority of observers considers petrous apicitis to be an osteitis that develops only in individuals with a pneumatized petrous apex. Communication of these air cells with the mastoid and middle ear is well documented. CT scans in this circumstance reveal debris within the petrous apex air cells and lysis of bony septa (Fig. 3.47, Fig. 3.48, Fig. 3.49, and Fig. 3.50). As such, petrous apicitis is analogous to coalescent mastoiditis. Disruption of the anterior or posterior bony cortex may occur and result in fulminant intracranial complications, such as meningitis, empyema, cranial neuropathy, and various other cavernous sinus symptoms. The initial diagnosis of petrous apex inflammatory disease is best made with high-resolution CT; however, after the diagnosis is made, MRI becomes important for evaluation of intracranial complications. Typical MR findings in these cases include pathologic enhancement at the periphery of the defect, presumably within the meninges, possibly extending to the gasserian ganglion (Meckel’s cave) and cavernous sinus.134 The clinical findings in patients with complicated petrous apicitis are well documented. The classic Gradenigo triad (otomastoiditis, sixth nerve palsy, and pain in the distribution of the fifth nerve) is actually rarely present; however, the patient may present with any one or more of these clinical entities.
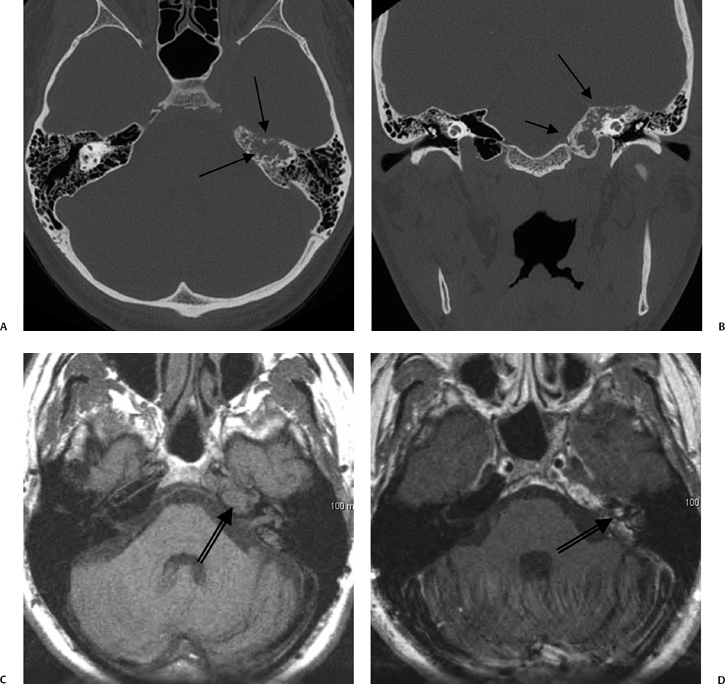
Fig. 3.47 Petrous apicitis. (A) Axial and (B) coronal computed tomography images demonstrate diffuse opacification localized to the petrous apex with loss of septations (arrows), indicating coalescent disease. (C) Axial precontrast T1-weighted magnetic resonance image (T1WI) reveals petrous apex disease (arrow). (D) Axial post-contrast T1WI reveals intensely enhancing debris. Leptomeningeal disease is manifest most notably by enhancement of the facial and superior vestibular nerves within the internal auditory canal (arrow).
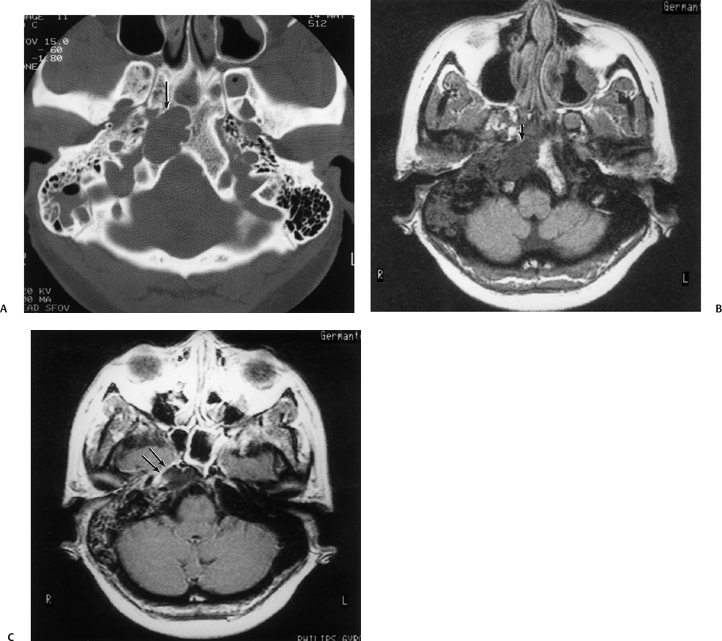
Fig. 3.48 Petrous apicitis. (A) Axial computed tomography (CT) image. There is diffuse debris throughout the right mastoid with multiple fluid levels. Well-marginated erosion at the right petrous apex (arrow) is detected. CT differential diagnoses include petrous apicitis and cholesterol granuloma, as well as recurrent cholesteatoma. (B) Axial noncontrast T1-weighted magnetic resonance image (T1WI). (C) Axial postcontrast T1WI. There is enhancement of the meninges (arrows) adjacent to the nonenhancing low signal intensity lesion in this patient with full Gradenigo syndrome.
Petrous apex lesions are considered in detail in Chapter 8. At this juncture, it is important to understand that benign debris is very common in the asymptomatic patient. In particular, trapped fluid occurs commonly as an incidental finding on both CT and MRI and should not be confused with clinically significant disease (Fig. 3.51 and Fig. 3.52).135 The imaging appearance of petrous apicitis is nonspecific, and the diagnosis must be based on clinical symptomatology and patient history. Differential diagnosis includes trapped fluid, mucocele, CH, cholesterol granuloma, and cephalocele. The latter, petrous apex cephalocele, represents a protrusion of meninges and CSF from Meckel’s cave and is typically an incidental finding. Meningocele and arachnoid cyst both fall under this umbrella.136
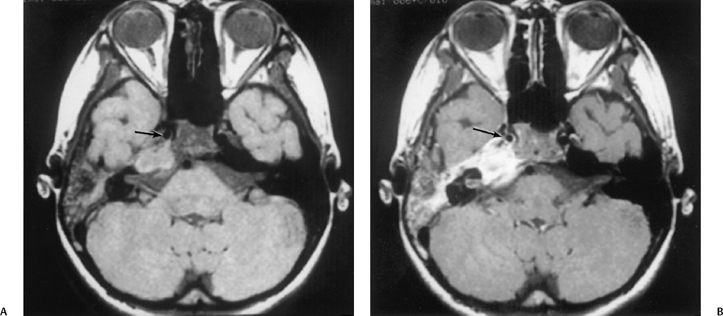
Fig. 3.49 Petrous apicitis. (A) Precontrast axial T1-weighted magnetic resonance image (T1WI). (B) Postcontrast axial T1WI. There is diffuse signal aberration involving the entire mastoid with obvious extent to the petrous apex. There is remarkable intense enhancement. There is anterior displacement of the cavernous carotid (arrow, A,B). Differential diagnosis includes aggressive neoplasms (including rhabdomyosarcoma). Clinical symptomatology (sixth and eighth nerve involvement) abated, and magnetic resonance findings regressed after antibiotic treatment.
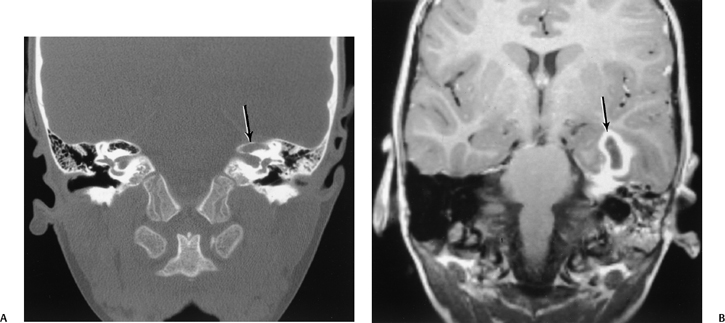
Fig. 3.50 Petrous apex infection. Abscess. (A) Coronal CT reveals debris at the left petrous apex with subtle evidence of bony erosion/permeation (arrow). (B) Corresponding coronal contrast-enhanced T1-weighted MRI reveals a mature abscess within the temporal lobe (arrow). Note the underlying enhancement at the petrous apex and diffuse debris throughout the mastoid.
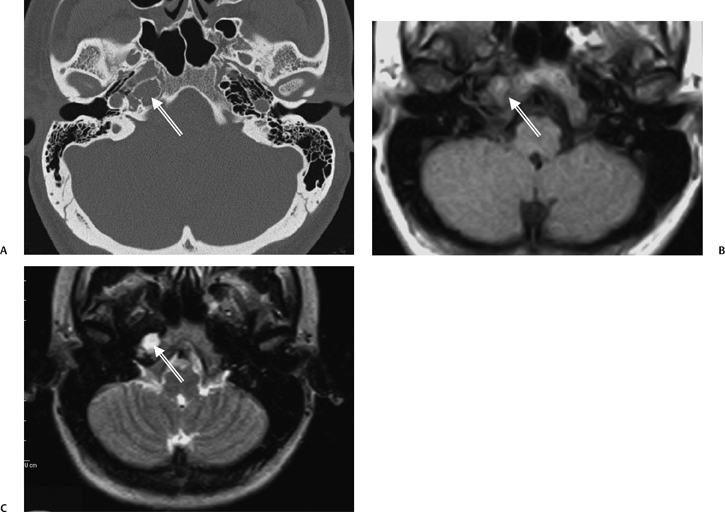
Fig. 3.51 Benign “trapped” fluid in the petrous apex. (A) Axial CT reveals debris (arrow) at the petrous apex, which is well corticated. (B,C) Axial T1-weighted MRI and T2-weighted MRI demonstrate homogeneous fluid intensity (arrows).
Chronic Otomastoiditis and Complications
As the name implies, chronic otomastoiditis is an unresolved inflammatory process of the middle ear and mastoid associated with active and quiescent periods. TM perforation is quite commonly associated. Virtually all of these patients have long-standing eustachian tube dysfunction, and subsequent decreased intratympanic pressure is most likely the basic predisposing factor for this variety of disease processes. Pseudomonas aeruginosa and Staphylococcus aureus are the most common organisms involved and are typically -lactamase-producing (penicillin-resistant).110,137–142
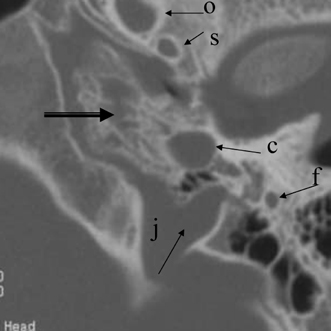
Fig. 3.52 Effusion, petrous apex. Axial computed tomography reveals opacification of petrous apex air cells (arrow) as an incidental finding in an asymptomatic patient. Note the normal foramen ovale (o), foramen spinosum (s), carotid canal (c), jugular foramen (j), and mastoid segment facial nerve (f).
All manifestations of COM, including CH, are unknown in subhuman primates. It has been postulated that the upright position and the enlargement of the skull in humans have altered the skull base so that the eustachian tube and tympanic bone assume a more vulnerable vertical position.143
The contralateral ear is very often abnormal in cases of long-standing COM, with estimates as high as 63%.144
The vast majority of these patients have a poorly pneumatized mastoid, a consistent manifestation of eustachian tube dysfunction. There are numerous clinical manifestations of chronic otomastoiditis, many of which are both visible and diagnosable with current imaging criteria.145–148 In this section, the following entities are reviewed and updated: middle ear effusion, granulation tissue (including cholesterol granuloma), middle ear atelectasis (TM retraction), acquired CH, noncholesteatomatous ossicular erosions, and postinflammatory ossicular fixation. TM perforations and various forms of myringitis will only receive passive reference because they are obvious otoscopically (in the absence of external canal occlusive disease) and can only be indirectly appreciated on CT.
Middle Ear Effusion
Middle ear effusion (MEE) is commonly associated with both AOM and COM, and the discussion in this section is arbitrary. In fact, MEE often occurs in the absence of clinically apparent inflammation especially in children due to the relatively horizontal orientation of the eustachian tube and the presence of adenoidal tissue. Adenoidal tissue may harbor various microorganisms, which predisposes the patient to superimposed inflammation via eustachian tube propagation. CT is usually not needed, as uncomplicated serous otitis can be monitored otoscopically while it is being treated medically or with tympanostomy tubes.142 Decreased intratympanic pressure secondary to eustachian tube dysfunction is the accepted etiology (Fig. 3.53, Fig. 3.54, Fig. 3.55, Fig. 3.56, Fig. 3.57, Fig. 3.58, and Fig. 3.59).142,149
The reader should be aware that 38% of individuals with untreated nasopharyngeal carcinoma have MEE. Auditory symptoms are the presenting complaint in 18% of these patients.150 This is presumably due to eustachian tube displacement or invasion. Tensor veli palatini invasion has been suggested as a specific functional cause.150
Therefore, unexplained middle ear effusion in an adult must be scrutinized, as it is a classic initial manifestation of nasopharyngeal neoplasm.142,151 These neoplasms often originate in the posterolateral recess (fossa of Rosen-müller) (Fig. 3.60 and Fig. 3.61). Trotter’s syndrome (unilateral deafness, trigeminal neuralgia, and soft palate immobility) may develop if there is associated perineural invasion.152 The nasopharynx is always in the field of view when the middle ear is examined. This provides the imaging specialist with a unique opportunity to make the initial diagnosis.
Effusions localized to the epitympanum (attic), mastoid antrum, and/or mastoid air cells (sparing the middle ear cavity proper) may be due to a selective decrease in intratympanic pressure in this area (“attic block”), that is, obstruction to ventilation of the attic, antrum, and mastoid air cells due to compromise of the anterior and posterior tympanic isthmi (see the Anatomy and Normal Variations section). This most commonly occurs in individuals with a developmentally small middle ear cavity and a poorly pneumatized middle ear cleft. The presence of a large Koerner septum (petrosquamosal lamina) also predisposes to this condition (Fig. 3.57).12,46,86,153 The reader should be aware that attic block may also result from the presence of a mass lesion (see also Fig. 3.25).
Middle ear effusions are diagnosed at CT by the presence of dependent radiopacity (see Fig. 3.53, Fig. 3.54, Fig. 3.55, Fig. 3.56, Fig. 3.57, Fig. 3.58, and Fig. 3.59).10 Effusions can also be appreciated with MRI, although fluid levels are of less significance as MRI is extremely sensitive and quite often findings are of no clinical significance.
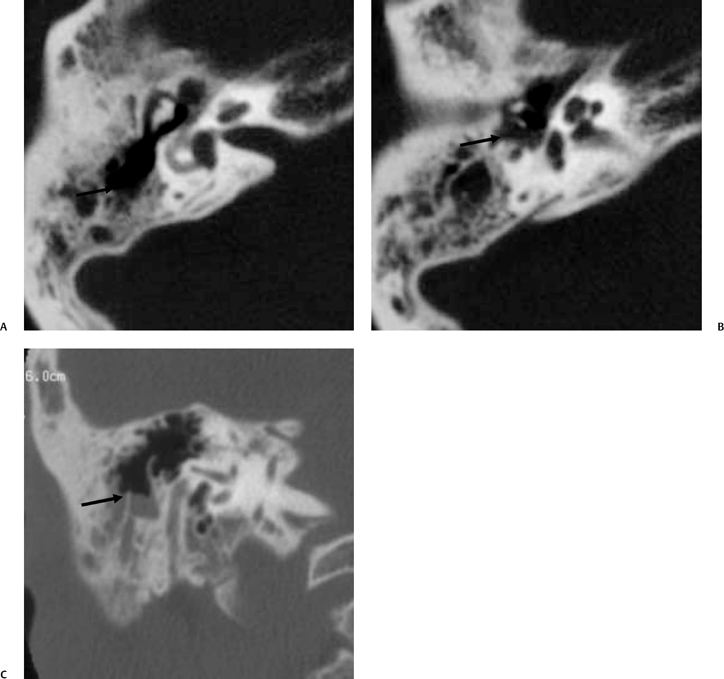
Fig. 3.53 Middle ear effusion. (A,B) Axial and (C) coronal computed tomography images reveal multiple fluid levels (arrows).
The purposes of surgically placed tympanostomy tubes are to normalize intratympanic pressure and limit the potential for developing superimposed bacterial infections (Fig. 3.62).92,149 A variety of materials are used, including various types of plastics and stainless steel.154,155 There are countless shapes and sizes produced by several manufacturers. Many of these have a bobbin shape, but some surgeons prefer a simple intratympanic phalange. The CT appearance of these devices is characteristic in most circumstances (Fig. 3.63, Fig. 3.64, Fig. 3.65, and Fig. 3.66).156,157 Identification may be hindered when there is surrounding fluid or when the tube is in an atypical location. These tubes must not be misidentified as a foreign body, dislocated ossicle, or inflammatory mass.156
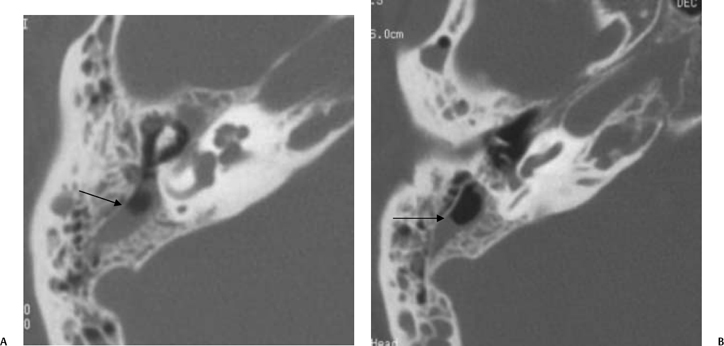
Fig. 3.54 Middle ear effusion. (A,B) Axial computed tomography images reveal well-defined fluid levels (arrows).
Tympanostomy tubes may become dislodged and extrude into the external canal or may actually migrate into the middle ear (Fig. 3.67).
Granulation Tissue (Typical and Cholesterol Granuloma)
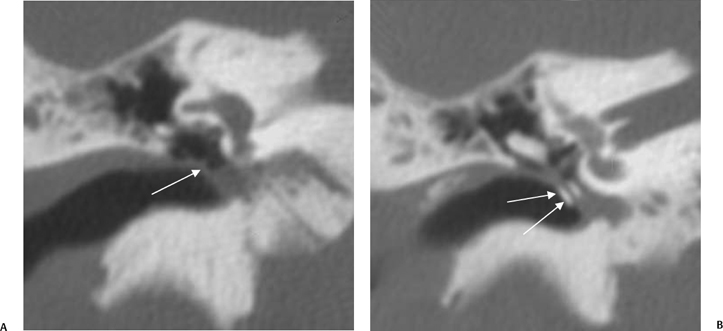
Fig. 3.55 Middle ear effusion. Coronal CTs reveal (A) fluid levels (arrow) and (B) a tympanostomy tube (arrows).
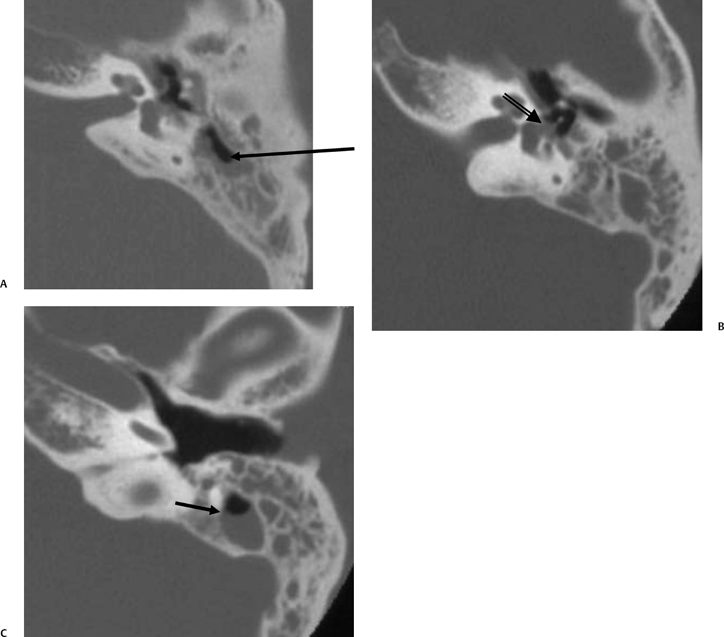
Fig. 3.56 Middle ear effusion. (A,C) Multiple axial computed tomography images reveal fluid levels (arrows). There is debris in the oval window niche surrounding the stapes superstructure (arrow, B), also a combination of granulation/fluid.
The development of granulation tissue (GNT) in the middle ear is extremely common, both as an isolated phenomenon and in conjunction with other middle ear maladies, such as effusion and CH.158 From an imaging point of view, one should consider granulation tissue as either typical or cholesterol granuloma. Both types appear at CT as nonspecific, nondependent radiopacity, although a few fluid levels may be present. Typical GNT is vascularized and enhances intensely with gadolinium on T1-weighted MR images. This is an extremely common entity. Cholesterol granuloma (CG), however, is far less common and has a distinct tendency to bleed, causing hemotympanum.158–161 As such, evaluation of enhanced T1-weighted MR images allows differentiation of typical GNT from CH (no enhancement with CH), and unenhanced T1-weighted MR images allow differentiation of CH from cholesterol granuloma (CG has bright signal on all pulse sequences).162,163 Enhancing (typical) granulation tissue is significantly more common than CH or cholesterol granuloma (Fig. 3.68 and Fig. 3.69). The presence of typical GNT can often be inferred indirectly in a patient with nonerosive middle ear debris appreciated on CT as CH will not usually result in at least some bone erosion (Fig. 3.70, Fig. 3.71, and Fig. 3.72).
Cholesterol granuloma is a cause of idiopathic hemotympanum.164 CG is a foreign body, giant cell reaction to cholesterol deposits with associated fibrosis and vascular proliferation. Pathologic examination reveals brownish fluid containing cholesterol crystals.160,165–168 Multinucleated giant cells are also apparent microscopically, as are red blood cells and hemosiderin. The cholesterol crystals and subsequent foreign body response are likely responsible for most histopathologic manifestations.169 Associated hemorrhage aggravates the circumstance, leading to additional neovascularity and continuing hemorrhage.168 Eustachian tube dysfunction (occlusion of the air cell system) with secondary mucosal edema and blood vessel rupture is considered the most likely etiology. Stagnation of these hemorrhagic contents occurs due to the lack of drainage. Red blood cell breakdown leads to further formation of the cholesterol crystals, which continue the cycle of granulomatous reaction. Important for the novice observer, cholesterol granuloma should never be confused with CH. CG and its variants are all lined by fibrous connective tissue in contradistinction to CH, which is lined by keratinizing stratified squamous epithelium.
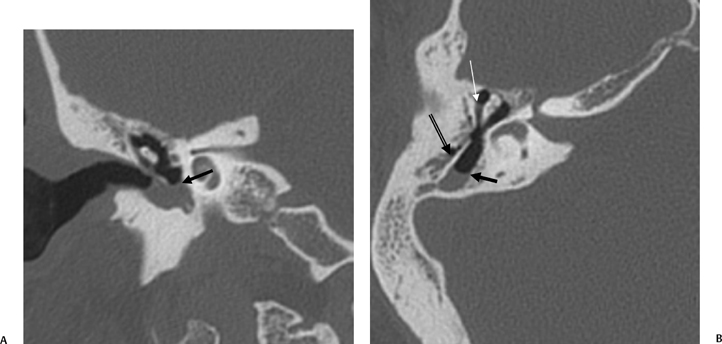
Fig. 3.57 Middle ear effusion. (A) Coronal computed tomography (CT) image reveals a fluid level in the middle ear (arrow). (B) Axial CT image reveals a fluid level in the mastoid antrum associated with an unusually thick Koerner’s septum (outlined arrow). There is also an incidental fluid level within Prussak’s space (white arrow).
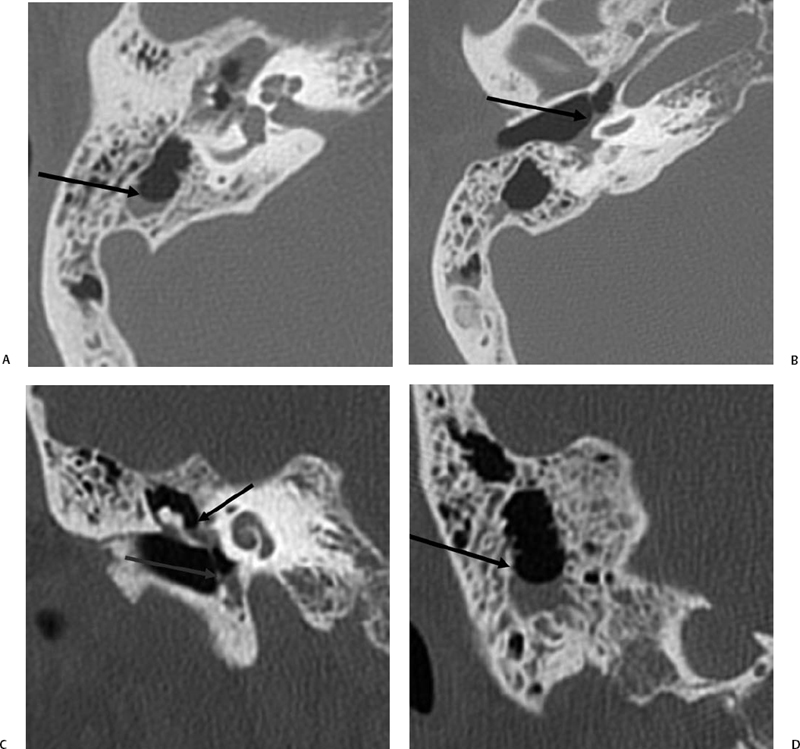
Fig. 3.58 Middle ear effusion. (A,B) Axial CTs reveal numerous fluid levels (arrows). (C,D) Coronal CTs reveal numerous fluid levels (arrows).
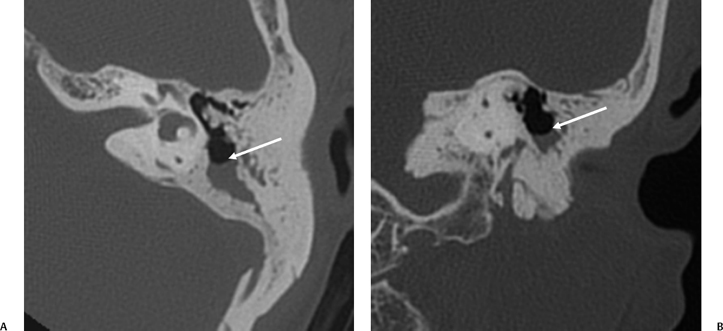
Fig. 3.59 Mastoid antrum effusion. (A) Axial and (B) coronal computed tomography images reveal well-defined fluid levels (arrows) within the mastoid antrum.
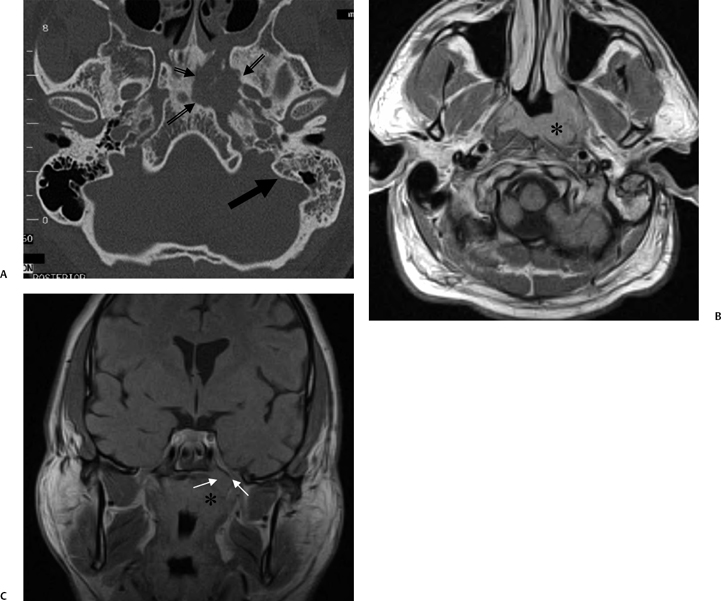
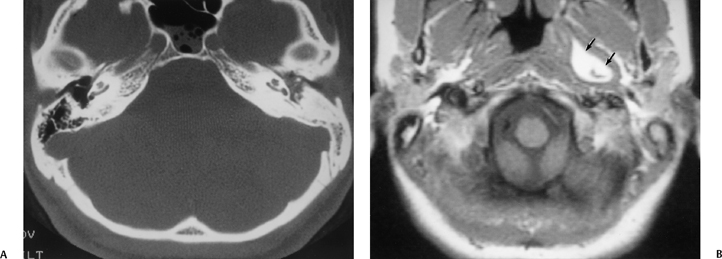
Fig. 3.61 Dermoid obstructing eustachian tube. (A) Axial computed tomography image. Diffuse nonerosive debris is seen throughout the left middle ear in a patient with a sclerotic mastoid. (B) Axial T1-weighted MRI reveals a high signal intensity mass (arrows) within the nasopharyngeal portion of the eustachian tube. (Case courtesy of William P. Dillon, MD.)
Fig. 3.62 Tympanostomy tube placement. (Used with permission, Salt Lake City ENT Associates.) (See Color Plate Fig. 3.62.)
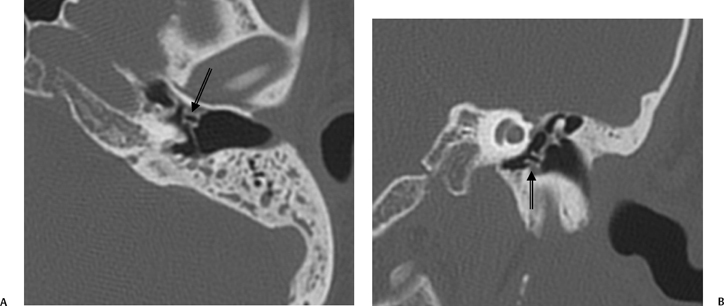
Fig. 3.63 Tympanostomy tube, anteriorly positioned. (A) Axial and (B) coronal computed tomography images. Tympanostomy tube is in place, but positioned more anteriorly than we typically see (arrows). Pressure equalization is usually still effective under these circumstances.
Cholesterol granuloma is usually preceded by chronic MEE. Superimposed bacterial infection may or may not be present. Causes are rarely erosive or destructive in most patients when they are isolated to the middle ear (Fig. 3.73). There has been one reported case of inner ear involvement secondary to middle ear CG.170 This is in contradistinction to CG of the petrous apex, which has the tendency to be extremely erosive and may compromise the carotid canal and cavernous sinus anteriorly or, less commonly, the cerebellopontine angle posteriorly.
Importantly, CG limited to the middle ear may masquerade otoscopically as a vascular mass, and the clinician may initially suspect paraganglioma (glomus tympanicum), aberrant ICA, or dehiscent internal jugular vein. The history of COM is usually the “tip-off” in this regard, and imaging will be diagnostic.
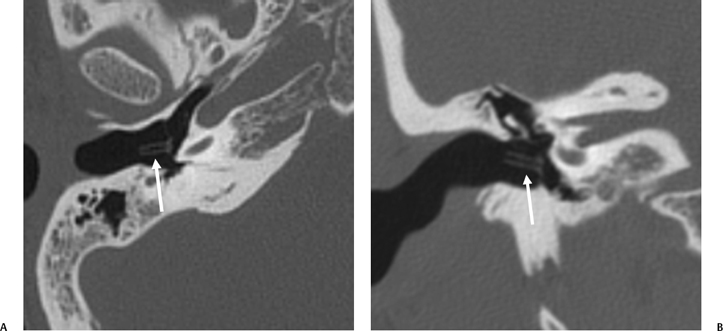
Fig. 3.64 Tympanostomy tube. (A) Axial and (B) coronal computed tomography images reveal phalange-shaped tympanostomy tube (arrows).
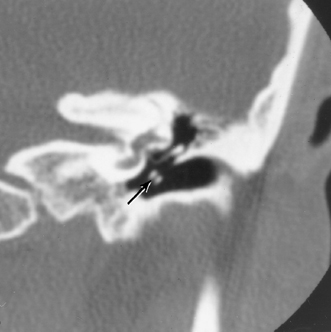
Fig. 3.65 Tympanostomy tubes (arrow). Coronal computed tomography image, nonmetallic bobbin-type tube.
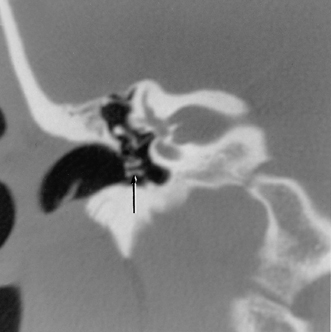
Fig. 3.66 Tympanostomy tubes (arrows). Coronal computed tomography image, nonmetallic device, right ear.
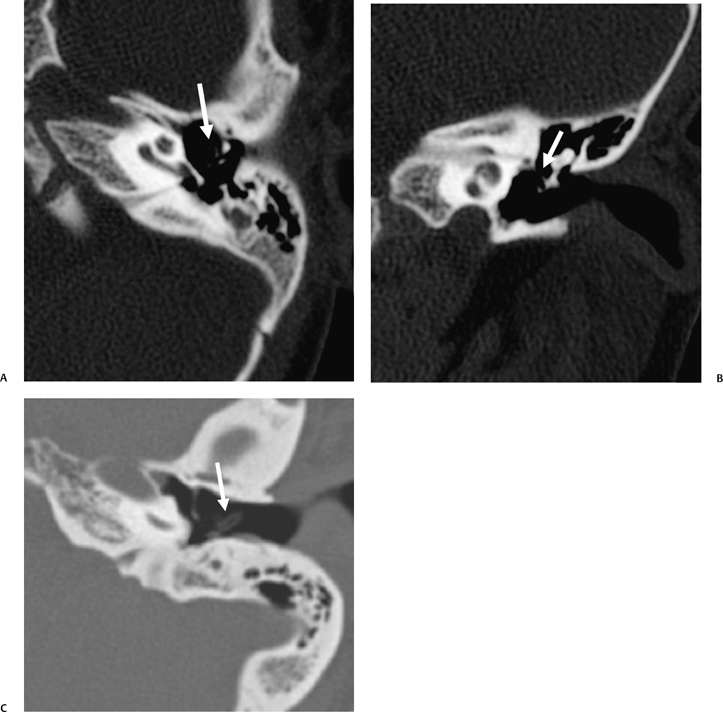
Similar to petrous apicitis, the risk of petrous apex CG is theoretically limited to only those 30% of patients who have a pneumatized petrous apex. Petrous apex CG is not common, yet the incidence is 10 times more common than CH in this region and 40 times more common than mucocele. By contrast, schwannoma of CN VIII (acoustic neuroma) is 30 times more common than CG.171 Petrous apex CG tends to remain clinically silent until there is encroachment on crucial structures, particularly the cranial nerves. Many patients have retrocochlear symptomatology due to involvement of the internal auditory canal. Others have headaches due to traction on the adjacent middle cranial fossa dura or facial pain due to the involvement of the posterior cranial fossa dura. Facial nerve involvement is rare. Surgical treatment is drainage and establishment of permanent aeration. Unlike CH, there is no true epithelial lining; therefore, complete excision is not necessary.172 Drainage is translabyrinthine in those with a “dead” ear and infralabyrinthine in others. Yearly surveillance is recommended to limit recurrences.
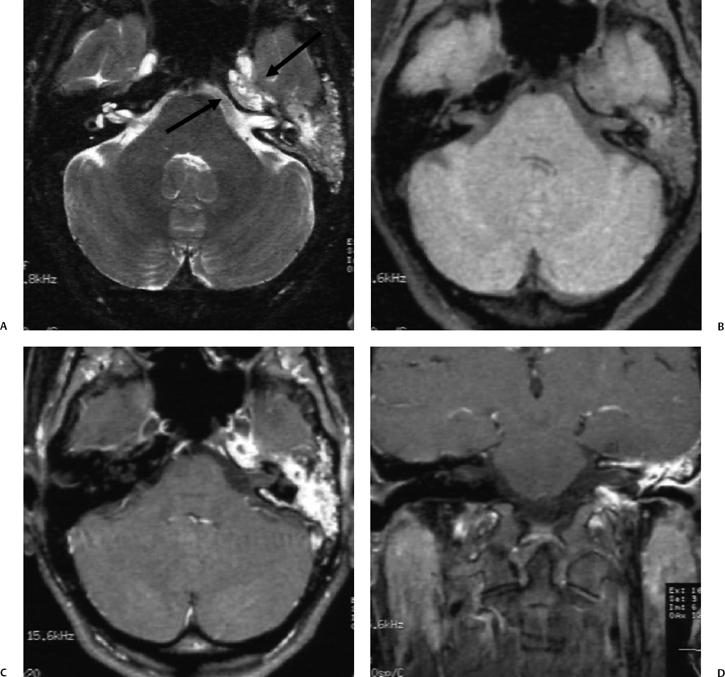
Fig. 3.68 Granulation tissue, typical (A) axial T2-weighted MRI reveals diffuse nonerosive (CT confirmed) hypersignal throughout the middle ear and mastoid extending to petrous apex (arrows). (B) Axial T1-weightedMRI (T1WI) confirms disease. (C) Axial and (D) coronal contrast-enhanced T1WIs reveal intense enhancement throughout, consistent with inflammatory granulation tissue.
Some confusing terminology is used in describing CG. These lesions are variously referred to as chocolate cyst, blue-dome cyst, and giant cholesterol cyst.147,165,173,174 Giant cholesterol cyst is a term used to describe the identical histopathologic lesion when it arises at the petrous apex in patients with a well-pneumatized middle ear and mastoid and no history of chronic otitis.173,175 Cranial neuropathies are particularly common with this variant (see Chapter 8). 175 Jackler and Cho have recently proposed a hypothesis for the development of petrous apex CG in this context.176 They propose that these individuals have an inherently deficient bony partition between the petrous apex air cell system and the adjacent marrow compartment. Hemorrhage from exposed marrow coagulates within mucosal cells and occludes outflow pathways. Sustained hemorrhage results in cyst expansion.
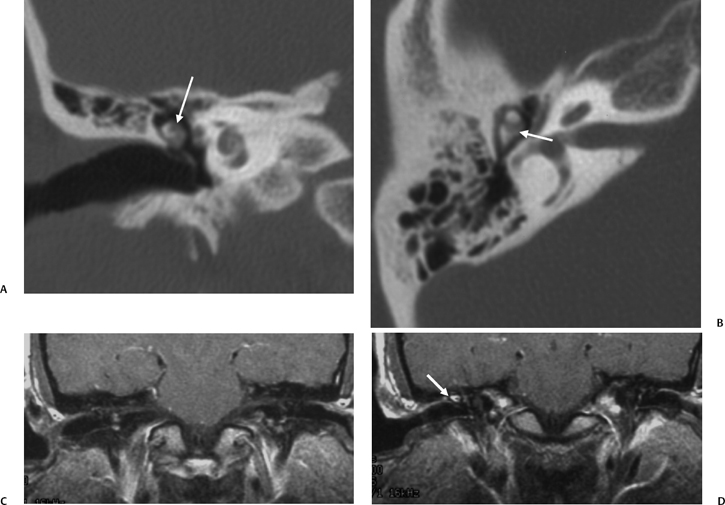
Fig. 3.69 Erosive debris, attic. (A) Coronal computed tomography (CT) image reveals soft tissue abnormality within the attic (arrow) eroding the malleus. The scutum is normal. (B) Axial CT image reveals a lesion eroding ossicles in the vicinity of the malleoincudal articulation (arrow). (C,D) Coronal pre- and postcontrast T1-weighted magnetic resonance images reveal enhancement (arrow, D), indicating granulation tissue rather than cholesteatoma. There was no surgery.
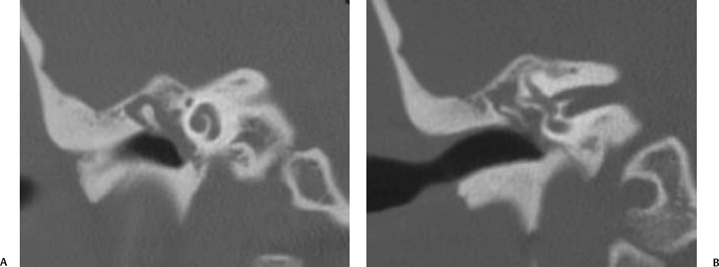
Fig. 3.70 Granulation tissue. Coronal computed tomography images through the (A) anterior cochlear turns and (B) vestibule demonstrate nonerosive, nonexpansile holotympanic debris consistent with benign granulation tissue. These findings are nonspecific, and glomus tympanicum paraganglioma can have this appearance.
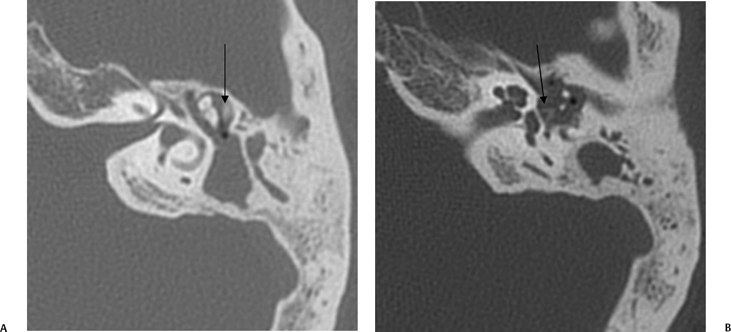
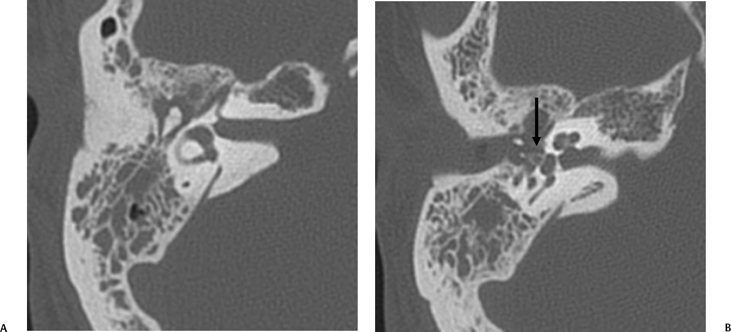
Fig. 3.72 Granulation tissue. (A,B) There is nonerosive debris throughout the middle ear cleft. The ossicular chain, including the stapes (arrow, B), is intact.
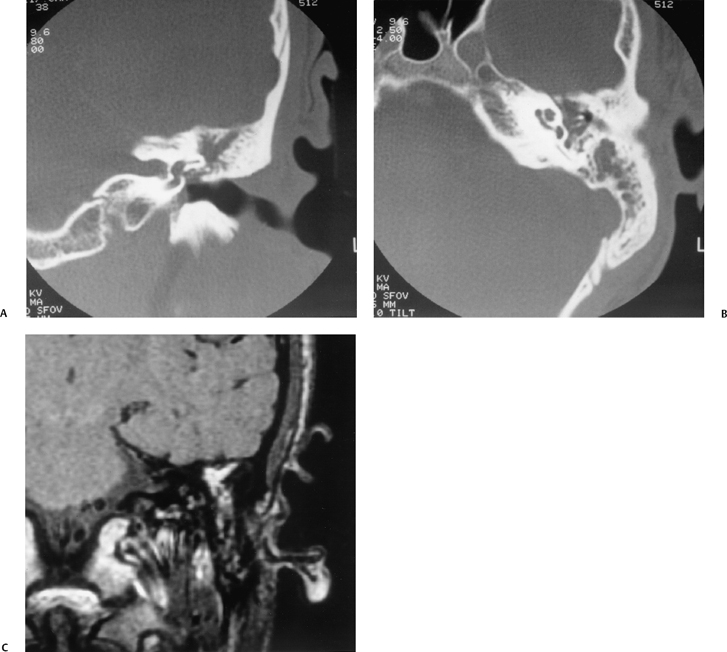
Fig. 3.73 Cholesterol granuloma, middle ear. (A) Coronal and (B) axial CTs. There is debris throughout the middle ear cleft. Note: Intact ossicles and retracted tympanic membrane indicate that this is very unlikely to represent cholesteatoma. (C) Coronal nonenhanced T1-weighted MRI. Bright signal is present, indicating methemoglobin (identical on T2-weighted MRI) and establishing diagnosis. See text beginning on page 98.
The terms chocolate cyst and blue-dome cyst refer to the identical histological entity occurring in a mastoidectomy cavity.
MRI is extremely helpful and in conjunction with CT is virtually diagnostic for CG (Fig. 3.74 and Fig. 3.75). With few exceptions these lesions are bright on all spin echo sequences, which differentiates them from CH; CH will usually have relatively long T1 and T2 relaxation times.147,165,174 Differentiation from normal marrow should not be difficult because fat demonstrates less intense signal as the repetition time (TR) is increased. Partial saturation gradient recalled echo sequences (GRASS), a technique that is sensitive to magnetic field inhomogeneities, may demonstrate a peripheral ring of low signal intensity that is almost certainly indicative of hemosiderin-laden macrophages in the fibrous wall. Proton chemical shift imaging may reveal a cyclic pattern of signal intensity centrally, reflecting the presence of lipid and cholesterol crystals within the lesion.165 These additional sequences may help to differentiate the hyperintense cholesterol granuloma from thrombosed aneurysm, hemorrhagic neoplasm, or atypical neuroma. A potential stumbling block in the differential diagnosis is the rare hydrated mucocele. The signal characteristics of these rare lesions may be very similar, presumably due to the high protein content (Fig. 3.76).
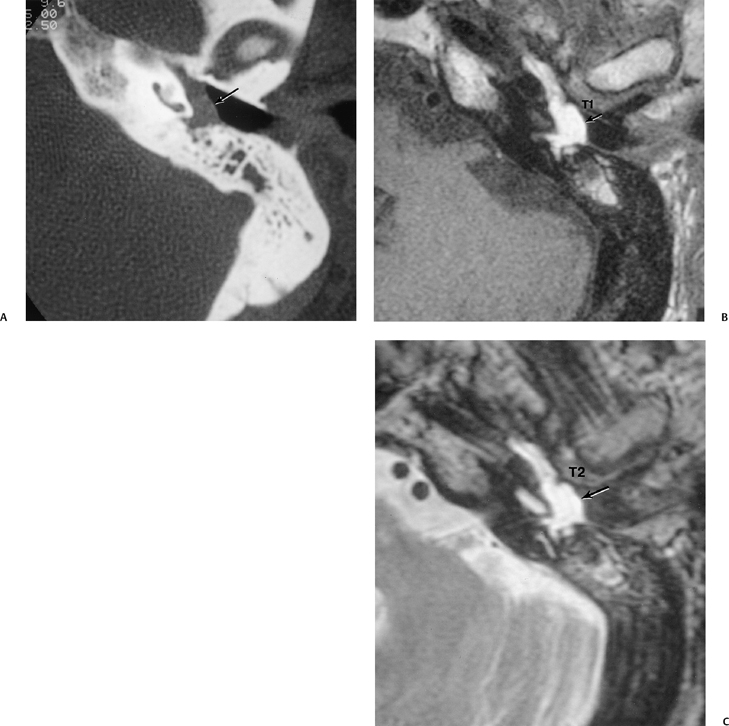
Fig. 3.74 Cholesterol granuloma, left middle ear. (A) Magnified axial CT, left ear. Debris is seen in the mesotympanic space (arrow). (B) Corresponding magnified axial noncontrast T1-weighted MRI. Debris is diffusely hyperintense (arrow). (C) Corresponding magnified axial T2-weighted MRI. Debris is hyperintense on this sequence as well (arrow). Findings are consistent with the presence of extracellular methemoglobin.
Petrous apex/cerebellopontine angle CG should not be confused with epidermoid congenital CH despite their smoothly expansile nature.166 MR signal characteristics in most cases are quite different from cholesterol granuloma/cholesterol cyst in that T1 and T2 relaxation times are both quite long. Petrous apex CG is not uncommon and has the classic appearance of a destructive lesion with MR characteristics of subacute/chronic blood (Fig. 3.77, Fig. 3.78, and Fig. 3.79). Differentiation of petrous apex CG from both trapped fluid and simple asymmetric pneumatization is a common clinical/imaging issue. Trapped fluid is very common and is easily differentiated from petrous apex CG; it is nondestructive and is characterized by long T1 and T2 relaxation times (Fig. 3.51 and Fig. 3.52). Asymmetric pneumatization may result in some confusion, as fatty marrow on the nonpneumatized side has similar hyperintensity to chronic hematoma (Fig. 3.80 and Fig. 3.81). Again, CT is confirmatory, as there is no bony alteration.
Fig. 3.75 Cholesterol granuloma, right ear. (A) Magnified coronal noncontrast T1-weighted MRI, right ear. (B) Magnified coronal T2-weighted MRI, right ear. Diffuse hyperintense debris throughout the middle ear space is virtually diagnostic of cholesterol granuloma.
Destructive petrous apex lesions may be subtle but extremely significant, as metastatic disease has a strong propensity to develop in this location due to the presence of vascular blood marrow (Fig. 3.82 and Fig. 3.83).
Petrous apex arachnoid cysts may appear identical to CH by both CT and MR criteria.177 Petrous apex mucocele is typically associated with long T1 and T2 relaxation times and perhaps very modest peripheral contrast enhancement (Fig. 3.84).178 Obliteration of the communicating cell tracts from the middle ear space is presumed causative. At CT, an expansile lesion with erosive margins may be present.179 A history of chronic otitis is variable.
Treatment of CG is effective surgical drainage. Such devices are well seen with CT (Fig. 3.85). MRI is also helpful in the postoperative evaluation of cholesterol granuloma. Successful surgical intervention will result in a dramatic decrease in T1 signal.165
Central Nervous System Complications
Central nervous system (CNS) complications are associated with acute otomastoiditis, as described earlier. CNS complications are also associated with chronic otitis media even in the absence of CH.180 There are three main categories: (1) abscesses and meningitis, (2) dural herniations and encephaloceles, and (3) CSF leaks. The presence of either category 2 or 3, of course, predisposes to category 1. Dural herniations and encephaloceles are well visualized with CT/MRI (Fig. 3.86). These are most commonly associated with prior mastoidectomy (vide infra), but they may also occur spontaneously often in association with long-standing COM. Labyrinthine fistula and facial nerve involvement are commonly encountered. CSF leaks may or may not be present. Most can be surgically corrected using a simple transmastoid repair. Idiopathic encephaloceles (without any predisposing condition) are rarely reported.181
Middle Ear Atelectasis
Although easily identified otoscopically, CT verification of TM retractions is usually simple, particularly when the membrane itself is thickened (Fig. 3.87 and Fig. 3.88). TM retractions may arise in either the pars flaccida or the pars tensa and are described clinically as mobile or fixed.182,183 Pars flaccida (attic) retractions are of particular diagnostic importance due to the propensity for developing associated acquired attic CH (Fig. 3.89 and Fig. 3.90).184–186 Many observers believe that this type of retraction may be due in whole, or in part, to associated obstruction of the anterior and posterior tympanic isthmi, which are responsible for ventilation of the attic, the antrum, and the remainder of the mastoid. Others dispute this concept.58 Although the retractions themselves were well visualized, CT is mainly ordered for ruling out small (or even large) CHs that may be hidden from the examiner’s view.148
Fig. 3.76 Hemorrhagic granulation tissue. (A) Axial computed tomography (CT). Nonexpanded petrous apex contains debris (arrow). Air cells are developed. (B,C) Axial and coronal T1-weighted magnetic resonance images. Mixed intensity lesion with bright signal periphery (arrow). Patient had sensorineural hearing loss (cause unknown) and a mild fifth nerve palsy. At surgery, only “brownish thin fluid” was found; there were no cholesterol crystals.
Pars tensa retractions are more common and may predicate the development of ossicular erosions, particularly in the vicinity of the incus long process (Fig. 3.91 and Fig. 3.92).11,13,187 Severe retractions extend to the cochlear promontory and in complicated cases may cause an ossicular discontinuity such that the TM becomes adherent to the capitulum of the stapes. This is referred to as nature’s myringostapediopexy due to its physiological similarity with surgically performed type 3 tympanoplasty. In these patients there is typically very little audiometric air/bone gap (conductive hearing will often be normal or near-normal) (Fig. 3.93). Middle ear effusions commonly coexist.
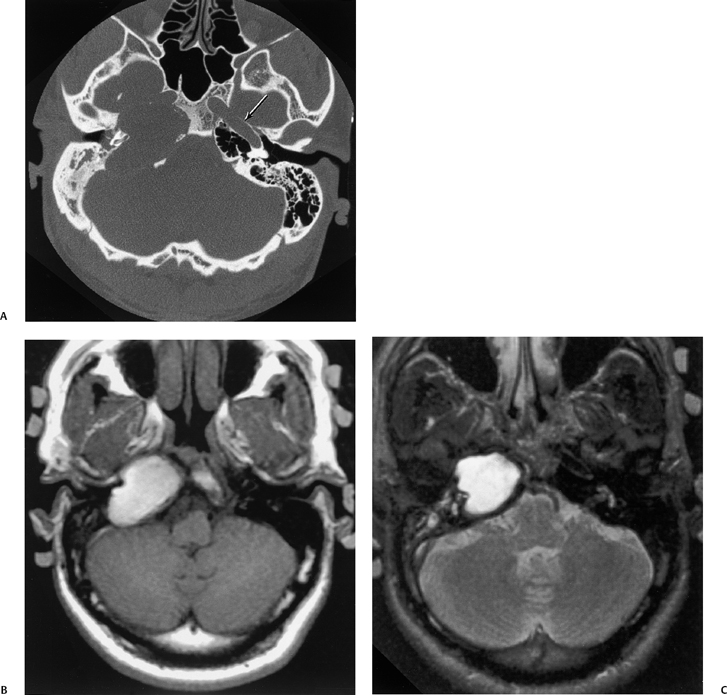
Fig. 3.77 Petrous apex cholesterol granuloma. (A) Axial computed tomography image. Large erosive mass at the right petrous apex. There is erosion of the carotid canal. Note the normal left carotid canal (arrow). (B) Nonenhanced axial T1-weighted magnetic resonance image. The mass is diffusely hyperintense. (C) Axial T2-weighted magnetic resonance image. The mass is diffusely hyperintense on this sequence as well. The hemorrhagic nature of the mass is indicated by the signal characteristics (extracellular methemoglobin). (Courtesy of Phillip S. Yussen, MD.)
Four clinical categories of TM retraction have been described: (I) retraction, (II) retraction with incus contact, (III) middle ear atelectasis, and (IV) adhesive otitis media.11 Adhesive otitis media refers to a condition in which the middle ear space is totally obliterated and the TM adheres to the ossicular chain and promontory, thereby obliterating mucosal surfaces (Fig. 3.94, Fig. 3.95, Fig. 3.96, and Fig. 3.97).188
Another report describes a type I retraction (no contact), type II retraction (contact with incudostapedial joint), type III retraction (contact with promontory), and type IV retraction (involvement of facial recess and sinus tympani). These observers recommend tympanoplasty for those with type II and type IV retractions.189
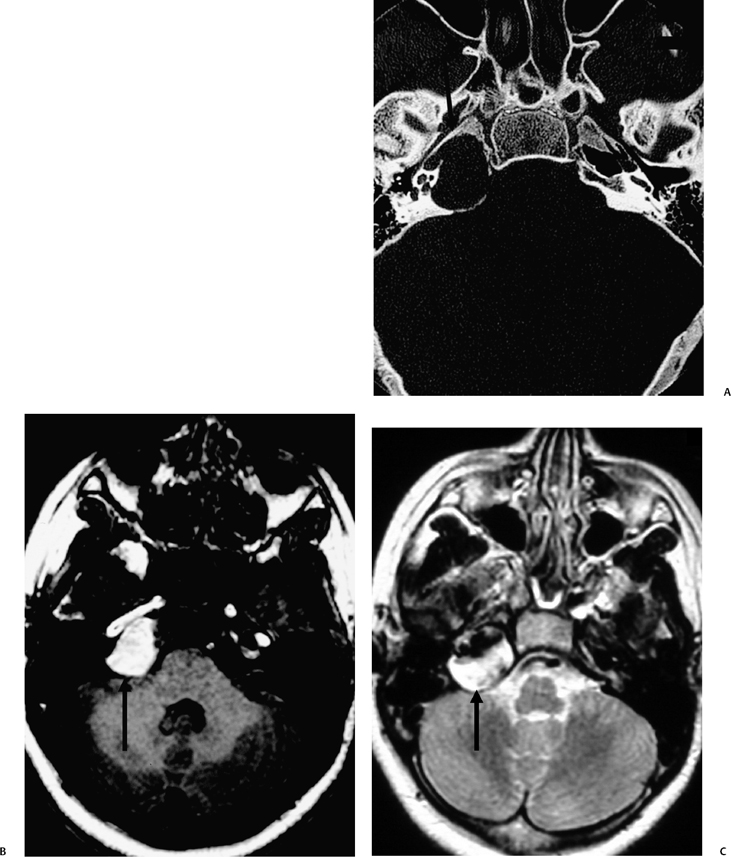
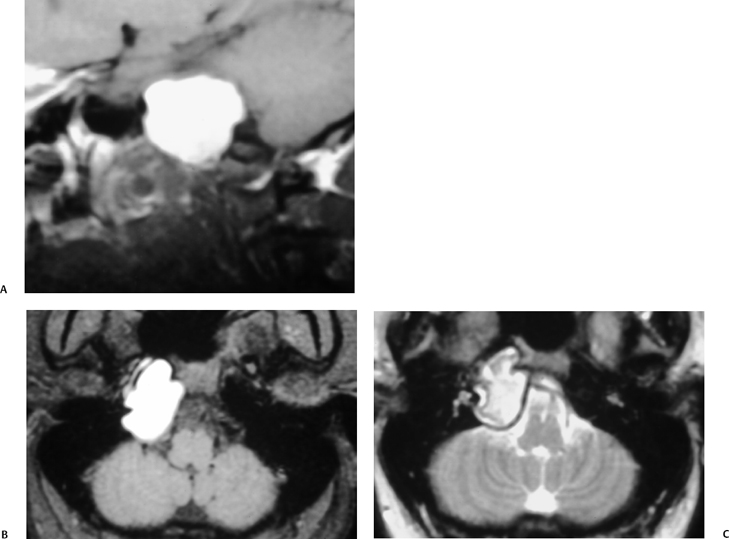
Fig. 3.79 Petrous apex cholesterol granuloma. (A) Axial and (B) sagittal T1-weighted MRIs (T1WIs), and (C) axial T2-weighted MRI reveal a hyperintense mass consistent with the hemorrhagic by-products visualized in this lesion. (Courtesy of Dave Yousem, MD.)
Acquired Cholesteatoma and Complications
CH (“skin in the wrong place”) is an expansile lesion of the middle ear or other pneumatized area of the petrous bone lined by keratinizing stratified squamous epithelium.141 CH is actually a misnomer because this lesion is not a neoplasm and may or may not contain cholesterol crystals. Many prefer keratoma, although this term has never been universally accepted.12,184
CHs may be congenital (epidermoid) or acquired (Table 3.6). About 98% of middle ear CHs are acquired. Congenital CHs are considered later in this chapter.
Acquired CHs arise from either the pars flaccida or the pars tensa of the TM and may be subdivided into primary acquired (no history of otitis media) and secondary acquired. There is likely a hereditary predisposition, and individuals of all ages are affected. Often, they are more invasive in young children, particularly when there is extensive mastoid pneumatization.191 The pars flaccida variety of CH is especially aggressive in children, although there is a relatively smaller incidence of this type compared with the adult. Both congenital and acquired CHs are more common in males.
| % | |
| Congenital | 2 |
| Acquired | 98 |
| Pars flaccida, usually primary acquired | 82 |
| Pars tensa, usually secondary acquired | 18 |
| Posterosuperior (sinus cholesteatoma) | 78 |
| Anterior and inferior | 22 |
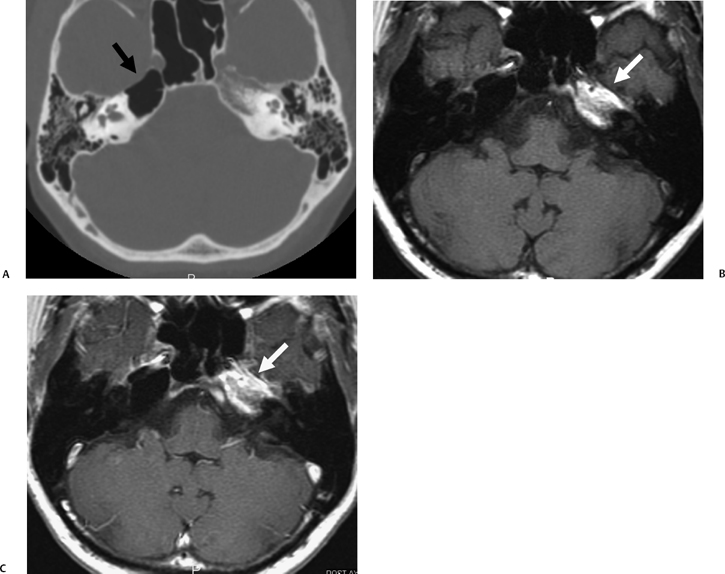
Fig. 3.80 Asymmetric petrous apex pneumatization. (A) Axial computed tomography image reveals a single large right petrous apex air cell (arrow). (B,C) Axial pre- and postcontrast T1-weighted magnetic resonance images reveal fatty signal within the normal marrow of the left petrous apex (arrows). (Courtesy of Ka-Khy Tze, MD.)
There is an increased incidence in patients with cleft palate, presumably secondary to the anatomic and physiological differences in these patients. Acquired CH is commonly associated with fibrous dysplasia when the external auditory canal is involved. Patients with bilateral CHs have a similar tendency toward recurrence as those with unilateral disease. Hearing preservation is often less successful in these patients.192
The etiology of acquired CH is a matter of ongoing debate. There are four major theories (Table 3.7): retraction, papillary proliferation, immigration, and metaplasia.193
| Retraction |
| Papillary proliferation |
| Immigration |
| Metaplasia |
Source: From Sudhoff H, Tos M. Pathogenesis of attic cholesteatoma: clinical and immunohistochemical support for combination of retraction theory and proliferation theory. Am J Otol 2000;21(6): 786–792. Reprinted with permission.
The retraction theory suggests that CHs arise from retraction pockets. As already indicated, eustachian tube dysfunction with decreased intratympanic pressure is believed to be responsible for TM retraction.11 The skin lining the EAC is unique. It lies directly on the periosteum of the EAC and on the external surface of the TM.141 The skin in this latter location grows faster and normally continuously migrates outward, with the cerumen produced in the lateral cartilaginous portion of the canal. Retraction pockets may remain dormant for many years, provided that normal physiological skin migration can occur. The safety of these retractions depends on this normal migration and physician cleansing.194 Otherwise, keratinized debris accumulates, an encrusted plug forms, and subsequent moisture causes expansion with the development of CH. Superimposed bacterial infection is a potential hazard because these lesions provide an excellent medium for bacteria.
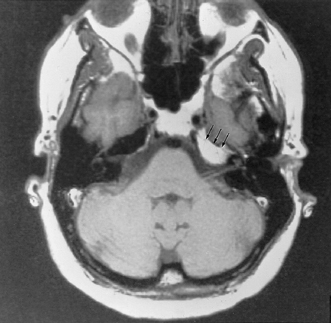
Fig. 3.81 Nonpneumatized petrous apex. Axial T1-weighted magnetic resonance image. Uniform bright signal (normal bone marrow) is present at the left petrous apex (arrows). Note the absence of mass effect. Computed tomography showed no air cell development.
Another proposed mechanism is papillary proliferation. This theory suggests that epithelial cones invade the subepithelial connective tissue via microscopic defects in the inner layers of the TM.11 This would explain why middle ear CH occasionally occurs without a clinically apparent defect in the TM or result in a secondary perforation. When the TM appears normal, these CHs are presumed by the clinician to be congenital in nature. In practice, it may not be possible to differentiate an acquired from a congenital CH in this context.
Recent evidence suggests that neither retraction nor papillary proliferation alone is a likely cause of the majority of acquired CH; presumably, the etiology in a majority of patients is a composite of these two theories.193
Proponents of the immigration theory believe that acquired CH results when keratinized stratified squamous epithelium from the surface of the TM invades the middle ear via a TM perforation.11,194 These patients have a history of repeated bouts of COM, which damages the internal mucosal lining of the TM, allowing stimulation of the outer keratinized squamous epithelium to invade the middle ear space and generate CH. There is much disagreement regarding the commonality of this process.59,193
The epithelial cells (both squamous and cuboidal) of the middle ear are pleuripotential and as such may undergo squamous metaplasia to keratinized squamous epithelium when stimulated by an inflammatory process.11 Although interesting laboratory substantiation of metaplasia as a cause for CH has been documented, there are few who espouse this theory.
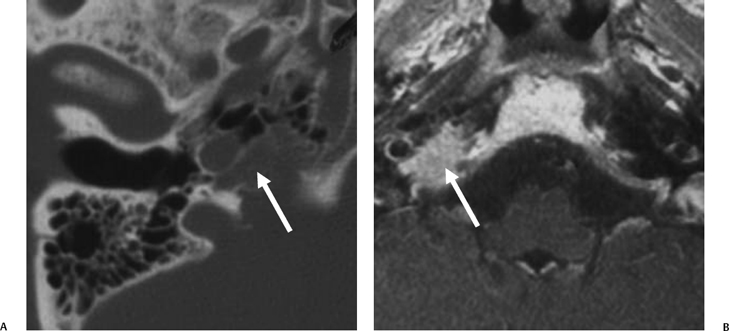
Fig. 3.82 Petrous apex metastatic disease. (A) Axial computed tomography image reveals a subtle destructive lesion involving the petrous apex (arrow). (B) Axial T1-weighted magnetic resonance image with contrast reveals intense enhancement of this lesion (arrow).
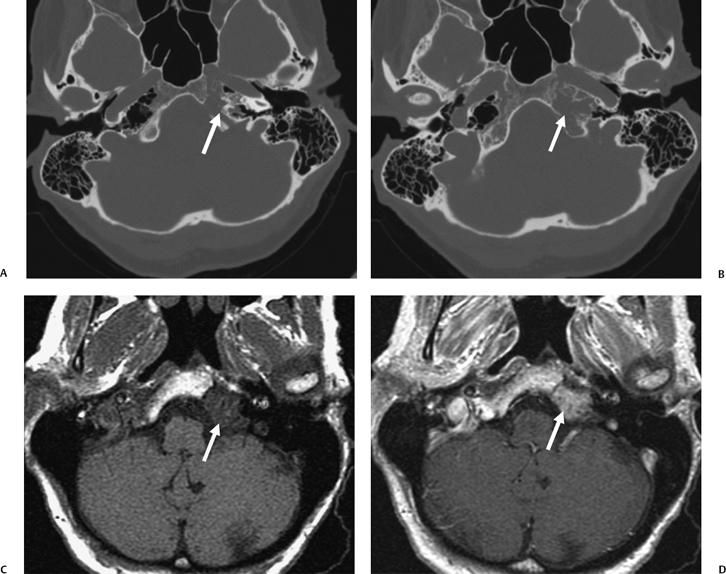
Most acquired CHs have typical growth patterns that depend on their site of origin. These pathways are strongly influenced embryologically by the four endothelial lined sacs previously described (see the Embryology section above).
Pars flaccida (attic) CHs begin in Prussak’s space (Fig. 3.98, Fig. 3.99, Fig. 3.100, Fig. 3.101, Fig. 3.102, Fig. 3.103, Fig. 3.104, Fig. 3.105, Fig. 3.106, and Fig. 3.107). This space opens posteriorly into the epitympanum. There is often medial displacement of the ossicular chain as the Prussak’s CH expands.182 Most commonly, the mass extends posteriorly via the superior incudal space to the posterolateral attic and subsequently further posteriorly through the aditus ad antrum into the mastoid antrum and central mastoid tract. A less common mode of extension is inferiorly via the inferior incudal space to the posterior tympanic recesses. This latter pathway is more common in children. Direct superior extension is ordinarily limited by the lateral malleal ligament. Rarely, extension is anteriorly and inferiorly into the protympanum, although the tensor tympani tendon is limiting in this regard. In fact, the mode of extension of attic CHs depends on the precise embryologic pneumatization of Prussak’s space, which is quite variable.9,12,195
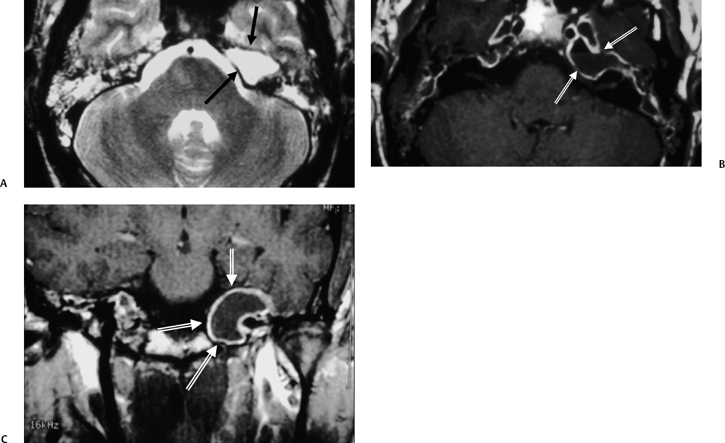
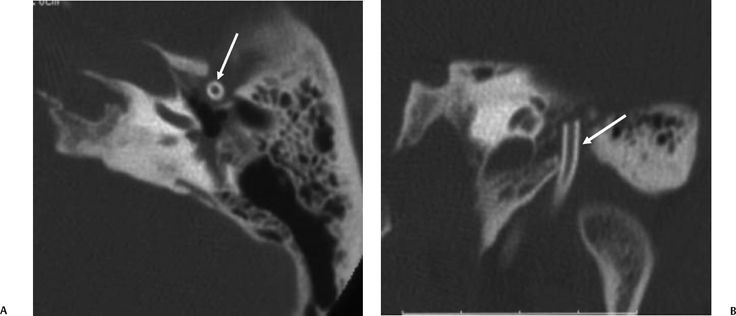
Fig. 3.85 Cholesterol granuloma, postexploration, drainage. (A) Axial and (B) coronal computed tomography images. Middle ear exploration revealed cholesterol granuloma within the anterior tympanic cavity. A drainage device (arrows) is in place, and there is no evidence of recurrence.
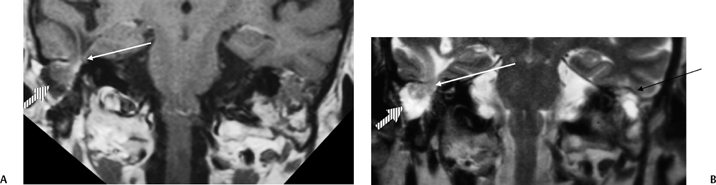
CHs arising from the pars tensa are usually due to posterosuperior retraction pockets (Fig. 3.108, Fig. 3.109, Fig. 3.110, and Fig. 3.111). These lesions will therefore begin in the posterior tympanum and commonly involve the posterior recesses, initially the more lateral facial recess and subsequently the more medial sinus tympani.196
When this medial extension occurs, they are often referred to as sinus cholesteatomas. Determination of the extent of these lesions is difficult to visualize otoscopically, but fortunately it is relatively simple with CT, particularly on examination of axial sections (Fig. 3.112). Surgical visibility is also a problem.197 Extension of these lesions is toward the mastoid antrum via the aditus. Many CHs arising in the pars tensa grow medial to the bulk of the ossicular chain and displace these structures laterally (Fig. 3.113).198
This is in direct contrast to Prussak (pars flaccida) CHs (described above), which typically pass lateral to the ossicular mass and displace it medially. CHs extending into the antrum consistently widen the aditus, often providing an important clue to diagnosis.198 CHs confined to the middle ear can give rise to chronic mastoid infection by inhibition of ventilation of the epitympanum secondary to occlusion of the anterior and posterior tympanic isthmi. One of the surgical goals in this circumstance is to lyse these adhesions to bring about normal ventilation of the entire mastoid. Rarely, CHs may develop anterior to the malleus head in the epitympanum (anterior epitympanic space). Facial nerve involvement at the level of the geniculate ganglion is quite common in this circumstance (Fig. 3.114).45
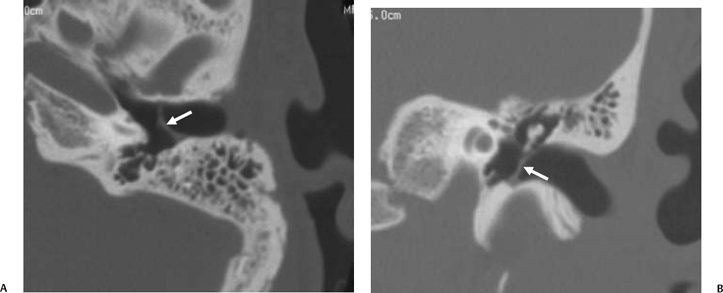
Fig. 3.87 Tympanic membrane retraction, pars tensa. (A) Axial and (B) coronal images reveal thickening and retraction of the pars tensa portion of the tympanic membrane (arrows).
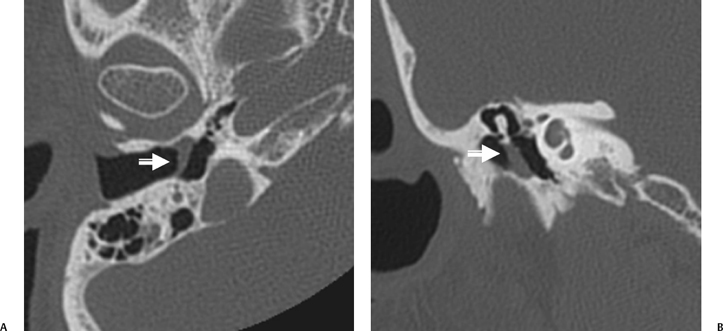
Fig. 3.88 Tympanic membrane retraction with thickening. (A) Axial and (B) coronal images reveal substantial thickening and retraction of the tympanic membrane (arrows). No middle ear debris is seen. The mastoid is somewhat sclerotic.
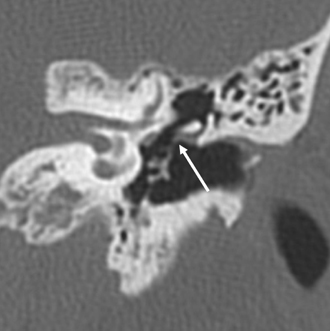
Fig. 3.89 Tympanic membrane retraction, pars flaccida. Coronal computed tomography image reveals a retraction involving the pars flaccida portion of the tympanic membrane (arrow). This patient also has a tympanostomy tube within a retracted pars tensa.
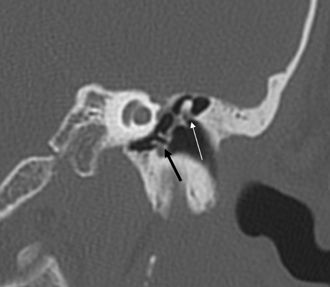
Fig. 3.90 Pars flaccida retraction. Coronal computed tomography image reveals retraction of the pars flaccida portion of the tympanic membrane (white arrow). Note the tympanostomy tube (arrow) is anteriorly positioned.
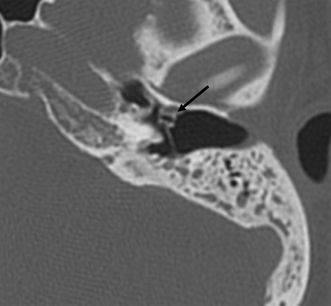
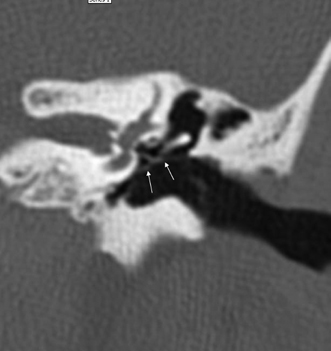
Fig. 3.92 Tympanic membrane retraction. There is broad retraction of the tympanic membrane (arrows) in this patient with long-standing chronic otitis. The ossicles are intact.
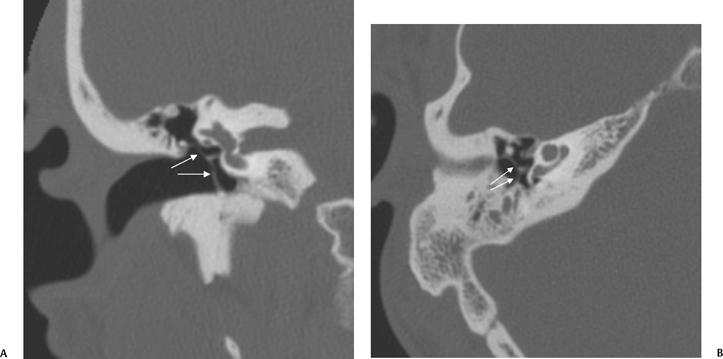
Fig. 3.93 Myringostapediopexy. (A) Coronal and (B) axial computed tomography images. There is broad retraction of the tympanic membrane (white arrows) to the head of the stapes. The long process of the incus is absent.
Petrosal CH, defined as CH medial to the otic capsule, may be congenital or acquired.199 This distinction is academic, as the behavior is identical. There are five subgroups: supralabyrinthine, infralabyrinthine, massive labyrinthine, apical, and infralabyrinthineapical.200 The term petrosal
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree