CHAPTER 23 Urologic and genital systems
Percutaneous nephrostomy (online cases 24 and 36)
Anatomy
The main renal artery and vein lie anterior to the renal pelvis. However, a posterior branch of the renal artery courses behind the renal pelvis to supply the dorsal segments. A percutaneous nephrostomy (PCN) track extending directly into a calyx has the least chance of causing substantial arterial injury (Fig. 23-1). Punctures into an infundibulum or directly into the renal pelvis carry a higher risk of hemorrhagic complications, because larger arterial branches may be traversed. Direct renal pelvic punctures also risk the formation of a urinoma after the tube is removed.
The calyces are usually arranged in anterior and posterior rows. At the upper and lower poles, fusion can result in compound calyces. During urography in the anteroposterior projection, classic teaching is that posterior calyces are located medially and seen en face, while anterior calyces are located more laterally and seen in profile. However, a recent evaluation of calyceal anatomy on computed tomography (CT) showed that this is not the case in the lower pole. Most lower poles have two to three calyces and the most posterior calyx was often more lateral.1 However, normal variation occurs, which is why, with the patient in prone position, oblique imaging, ultrasound, or injection of air is recommended to identify posterior calyces. Discrimination between the anterior and posterior calyces is critical, because posterior calyces are strongly preferred for most PCN procedures.
Understanding the normal and variant relationships of the kidneys with surrounding structures is important for safe and successful placement of a PCN.2 The descending colon lies in the anterior pararenal space, and its relationship to the left kidney is determined by the position of the lateroconal fascia. In approximately 10% of patients, the descending colon lies behind a horizontal line at the posterior edge of the left kidney. In about 1% of cases, the descending colon extends behind the left kidney.3 Rarely, the ascending colon lies posterolateral to the right kidney and medial to the liver.
The liver is anterolateral to the upper pole of the right kidney. In some patients, a portion of the right lobe of the liver may extend posterolaterally to the upper pole of the kidney. The position of the spleen is more variable. It typically lies superolateral to the kidney but often is adjacent to the lateral margin and frequently may be posterolateral to the upper pole. Because of these variants of colonic, hepatic, and splenic anatomy, review of prior cross-sectional imaging studies is critical before PCN.
Patient selection
The indications for PCN fall into four broad categories (Box 23-1). Each patient must be considered individually, because some have both indications for and contraindications to the procedure. The decision about whether to perform PCN depends on the details of the particular situation and on the other available options.
Box 23-1 Indications for Percutaneous Nephrostomy
Relief of urinary obstruction
In any patient with obstruction and suspicion of infection, emergent decompression is indicated. Infection may be suggested by fever, leukocytosis, flank pain, or sepsis. These patients must also be treated immediately with appropriate intravenous (IV) antibiotics and hemodynamic support as needed. The decompressive procedures in patients with pyonephrosis must be conducted and monitored with care, because the septic condition may be worsened with the intervention.
Patients with chronic unilateral hydroureteronephrosis due to malignancy do not necessarily require decompression unless infection is suspected. In the absence of malignant disease, kidneys with an estimated renographic glomerular filtration rate (GFR) of greater than 10 mL/min/1.73 m2 will likely stabilize or improve renal function, while kidneys with worse function may not be worth salvage.4 The finding of mild or moderate cortical atrophy on CT or ultrasound is not a reliable predictor of limited potential for functional recovery after a PCN. In fact, decompressive PCN may be indicated to assess the recoverable function of a chronically occluded kidney.
Diversion of urine
PCN is used to divert urine in cases of urinary leakage (Fig. 23-2). PCN, usually in combination with ureteral stent placement, allows most ureteral injuries to seal. The stent is thought to serve as a scaffold for ureteral healing. Percutaneous drainage of associated urinomas that accumulated before urinary diversion also may be performed. In cases of malignant or inflammatory fistulas, PCN is indicated to divert urine in conjunction with stent placement. PCN has been used also to divert urine and treat patients successfully with hemorrhagic cystitis.5
Access for diagnostic interventions
Percutaneous access may be performed to permit antegrade pyelography. However, advances in diagnostic radiology dominated by CT and magnetic resonance (MR) urography have rendered this indication extremely rare.6 Biopsies of urothelial lesions may be performed through nephrostomy access using brushes and forceps. Nephrostomy access may be used for diagnostic nephroscopy and ureteroscopy. Functional studies (e.g., Whitaker test) can be performed to determine the significance of urographically detected stenoses and to assess the results of interventions such as endopyelotomy or balloon ureteral dilation. This procedure is typically reserved for complicated cases in which nuclear scintigraphy or MR urography fail to give adequate physiologic information.7 MR urography may become the “one-stop shop” for anatomic and functional information regarding the genitourinary system.
Access for therapeutic interventions
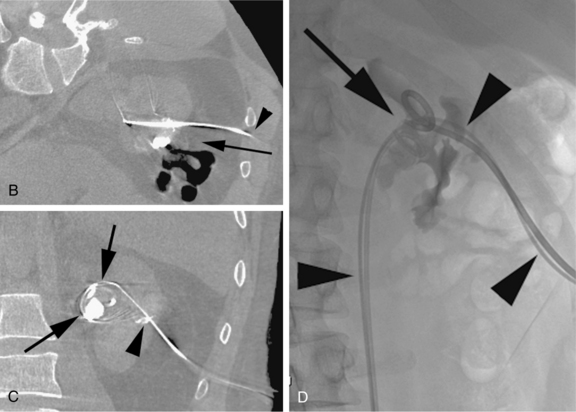
Ureteral strictures may be dilated percutaneously, and ureteral stents can also be placed. Foreign bodies (e.g., encrusted or occluded ureteral stents) may be retrieved through the nephrostomy access (Fig. 23-3). Stone therapy can be performed by chemodissolution or mechanical fragmentation and extraction. Antifungal agents may be infused directly through the nephrostomy tube to treat fungus balls in the upper urinary tract.8,9 Nephroscopically guided operations can be performed, including resection of urothelial neoplasms and endopyelotomy. Intracavitary adjuvant chemotherapy can be administered through a nephrostomy in certain cases of transitional cell carcinoma.10
Access provided for stone treatment is one of the most common indications for PCN placement. By the age of 70 years, 11% of men and 5.6% of women will have a symptomatic urinary tract stone.11 Although stones smaller than 5 mm often pass without intervention, stones between 5 and 10 mm have variable outcomes, and stones larger than 10 mm will not pass without intervention.11 Many renal calculi can be managed successfully with extracorporeal shock wave lithotripsy (ESWL) or percutaneous nephrostolithotomy (PCNL), with success rates that rival open surgery but with significantly less morbidity, recovery time, and cost.
Planning the approach and entry site is the most critical step of the PCNL procedure and should be made by consultation between the urologist and interventional radiologist. The puncture site must allow the stone to be accessed and removed but not be made directly into the pelvis or infundibulum. This is one of the situations where occasionally supracostal access and/or access to an anterior calyx is optimal for future treatment (Fig. 23-4). If the initial puncture site is not ideal, it is better to make a new puncture in a more suitable position than to proceed with track dilation through which the stone cannot be removed. The best approach depends on several factors, the most important of which is the location of the stone burden.
Contraindications
Contraindications to PCN include uncorrectable coagulopathy and an uncooperative patient. If hyperkalemia is severe (i.e., potassium level greater than 7 mEq/L), hemodialysis should be performed to correct the electrolyte balance before the procedure.
Surgical and medical alternatives
It usually is advantageous to initially consider a retrograde approach for renal drainage in patients with obstructive uropathy. PCN is typically reserved for patients in whom retrograde attempts are unsuccessful or not feasible. Although retrograde stents need routine changes and management, the patient will not have an external tube to manage. A “surgical” nephrostomy may be placed during open surgery directly into the renal pelvis, but this is rarely indicated because PCN can be performed safely and effectively in almost all patients (Fig. 23-5). Medical therapy for obstructive uropathy is limited. In some patients with widely disseminated malignancy or retroperitoneal fibrosis and obstructive uropathy, renal function may improve with the administration of corticosteroids.12,13
Technique
Preprocedure care
Prophylactic antibiotics should be administered intravenously before the procedure. The most common urinary tract pathogens are Escherichia coli, Klebsiella, Enterococcus, Proteus, and Staphylococcus species.14 For prophylaxis in the absence of any overt infection, a third-generation cephalosporin or a fluoroquinolone is usually chosen. Ciprofloxacin is commonly used in both oral and IV forms. When infection exists, therapy is directed toward the isolated organisms. Another option for broad coverage would be ampicillin with gentamicin.14,15
Procedure
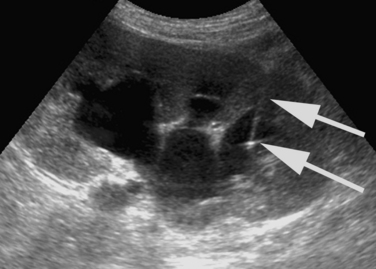
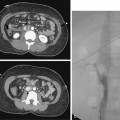
The initial puncture for PCN is performed with ultrasound, fluoroscopy, or very occasionally with CT imaging. Ultrasound is the optimum modality. A suitable calyx can be selected and entered with continuous sonographic guidance while avoiding surrounding organs (Fig. 23-6). Fluoroscopy can be used when renal function permits the IV administration of contrast material and results in satisfactory opacification of the collecting system. Fluoroscopy also may be used to puncture directly onto a radiopaque calyceal calculus (see Fig. 23-4). If a retrograde ureteral catheter has been placed, it may be used to opacify the collecting system. CT guidance for puncture occasionally may be required in patients with congenital anomalies (i.e., horseshoe or pelvic kidneys) or issues with body habitus or positioning (e.g., severe scoliosis, morbid obesity) (Fig. 23-7).
The ideal skin entry site is at least 12 cm lateral to the midline but medial to the posterior axillary line. If the skin entry site is too medial, the paraspinal muscles are traversed, and subsequent interventions are rendered more difficult because the guidewire and catheter must abruptly turn medially on entering the calyx. A medially placed nephrostomy also is more painful for the supine patient. If the skin entry is too lateral, the risk of colonic perforation increases. However, this risk is typically very small, estimated at up to 3% when the expected path during placement of lower pole nephrostomy tube is simulated using multiplanar CT.16
A posterolateral approach, with the needle angled about 30 to 40 degrees from vertical, directly into a posterior calyx is favored (see Fig. 23-1). This route follows the relatively avascular posterolateral plane of the kidney (i.e., Brödel line). Punctures generally should be made directly into a posterior calyx (see Fig. 23-1). This choice provides a straight vector into the renal pelvis and minimizes the amount of renal parenchyma traversed by the nephrostomy track. Infundibular punctures or punctures directly into the renal pelvis should be avoided because large arteries may be traversed. Inadvertent puncture of anterior calyces makes subsequent interventions more difficult because of the tortuous pathway and increases the risk for significant arterial injury. Anterior calyces should be targeted only in the rare patient with an anterior calyceal stone (see Fig. 23-4). Oblique imaging and injection of air or carbon dioxide is helpful to determine whether a calyx punctured under fluoroscopy is posterior or anterior (Fig. 23-8).
The initial puncture site is carefully scrutinized before dilation and catheter placement. If instillation of contrast or air reveals the initial puncture is not into a suitable posterior calyx, the needle should be left in place to opacify the system while a new needle is guided fluoroscopically into an appropriate calyx (Fig. 23-9). After the puncture is deemed satisfactory, a 0.018-inch guidewire is advanced into the renal pelvis and ideally down the ureter. A skin nick is made along the needle track. Blunt dissection of the subcutaneous tissue is performed. A coaxial access set, such as the Accustick set (Boston Scientific), is advanced under fluoroscopic guidance over the wire into the collecting system (see Fig. 23-8C). Urine can be aspirated and contrast injected through the sidearm of some access sets. The inner portions of the access set are removed. A 0.038-inch guidewire is advanced through the outer portion of the access set into the renal pelvis or ureter. In some access sets, the 0.018-inch wire can be left alongside the 0.038-inch wire as a “safety wire.” An angled guiding catheter may help direct the guidewire down the ureter into a more secure position if necessary (Fig. 23-10).
The track is then dilated, and an 8- to 12-French (Fr) locking pigtail nephrostomy catheter is placed. The pigtail is formed and locked within the renal pelvis (see Fig. 23-8E). Care must be taken to avoid forming the pigtail in the ureter or in a calyceal infundibulum. The 8-Fr tubes are satisfactory in patients with clear urine. Larger tubes may be helpful in patients with grossly purulent urine or stone debris. Locking pigtail catheters generally are preferred over other self-retaining catheters (e.g., Malecot, accordion, Foley). The nonpigtail catheters occasionally may be useful in collecting systems not capacious enough to accommodate a pigtail. In patients for whom access was provided for PCNL, a 5-Fr catheter is often left in the bladder alongside the nephrostomy tube, giving the urologist a stable access for a stiff working wire as well as access to the renal pelvis from the nephrostomy tube.
The catheter is secured to the skin using adhesive dressings or suture. Adhesive dressings minimize catheter kinking and reduce the risk of catheter dislodgment. After the track has matured, a simple dry dressing may be placed over the exit site. The catheter is left to gravity drainage with close monitoring of urine output. It typically requires irrigation only if blood clots occlude the tube.
Patients should be followed closely after the procedure for hemorrhagic or septic complications. In patients with bilateral obstructive uropathy undergoing PCN, dramatic fluid and electrolyte shifts may occur with postobstructive diuresis. In carefully selected patients, PCN may be performed safely as an outpatient procedure.17 In this situation, however, the operator should have a low threshold for admitting the patient if there is any evidence of hemorrhage, sepsis, or severe pain during or after the procedure.
Catheter maintenance
Colonization with bacteria, fungi, or both invariably develops in patients with chronic indwelling nephrostomy tubes.18 Asymptomatic bacteremia occurs frequently (about 10% of the time) during routine catheter exchanges; preprocedural antibiotics are not successful at preventing bacteremia.19 Nonetheless, some operators routinely give antibiotics for tube exchanges, particularly when the tube has been functioning poorly. For patients with cardiac valvular disease, a full course of antibiotics should be administered before catheter exchange to minimize the risk of bacteremia. Despite therapeutic levels of antibiotics during catheter exchange, a septic reaction may still occur as a reaction to endotoxin released into the vascular system.
Results
In patients with obstructed, dilated collecting systems, a decompressive PCN can be placed successfully in almost all cases. Technical success should be expected in greater than 95% of cases.20,21 In nondilated collecting systems or complex stone cases, technical success may decrease to 85% or less.
The clinical response to PCN by patients with urosepsis often is dramatic. In one series of patients with gram-negative septicemia and urinary obstruction complicated by infection, PCN reduced mortality from 40% to 8%.22 However, PCN in patients with pyonephrosis may exacerbate or precipitate septicemia as a result of bacteria entering the bloodstream through peripapillary veins from catheter manipulation or overdistention.22,23
In patients with fungal urinary infections, antifungal agents can be infused directly into the collecting system. This approach is advantageous because effective therapeutic levels can be achieved in the urinary tract without the associated systemic toxicity from IV administration. Fungus balls can be disrupted mechanically and extracted through the nephrostomy tract.8,24
In most patients with azotemia due to obstruction, PCN provides rapid improvement of renal function.25 The procedure commonly is used as a temporizing measure to improve renal function while the underlying obstruction is addressed definitively. In patients with terminal malignancies and azotemia due to bilateral obstruction, unilateral PCN usually suffices to preserve renal function.26 Bilateral drainage is indicated if pyonephrosis is suspected or unilateral drainage is unsatisfactory for restoring renal function. Unfortunately, the median survival rate of patients with advanced malignant urinary obstruction is 3 to 7 months.27 Even with careful patient selection, 32% of patients are unable to achieve any improvement in quality of life after PCN.28 After an open and frank discussion about the physical, emotional, and financial burdens associated with a drainage catheter, most patients and families opt for the drainage catheter as a means to extend life for as little as a few months.
Complications
The mortality rate for PCN is about 0.2%, which compares favorably with the mortality rate for surgical nephrostomy (≤6%).20 The major procedure-related complications are bleeding and sepsis. Hemorrhage requiring transfusion or other treatment occurs in 1% to 3% of patients undergoing PCN.20 One series of 144 nephrostomies placed using combined CT and fluoroscopic guidance had no hemorrhagic or other major complications.29 Most bleeding associated with PCN is transient and self-limited. It is not uncommon to have pink urine for several days after the procedure. If small clots block the catheter, the catheter should be irrigated with sterile saline.
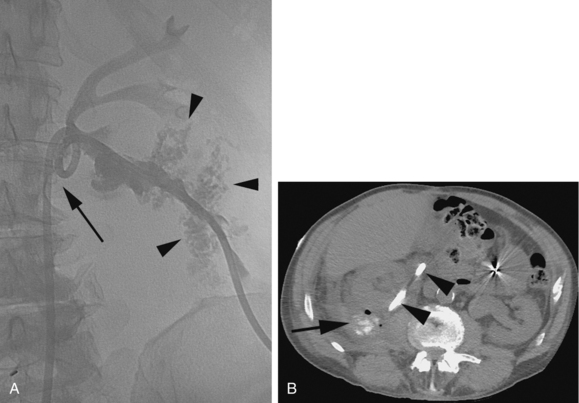
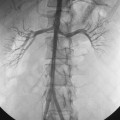
Major arterial injury should be suspected when the urine remains grossly bloody after 3 to 5 days, when new clots are demonstrated in the collecting system on follow-up nephrostograms, or when there is a significant drop in hematocrit. These patients should undergo angiographic evaluation with embolization of injured vessels (Fig. 23-11). If the initial angiogram is negative, the nephrostomy may be removed over a guidewire to relieve the tamponading effect of the catheter, and the angiogram is repeated. If the drop in hematocrit is out of proportion to the quantity of blood in the urine, the patient may have a retroperitoneal hematoma. Retroperitoneal hemorrhage is best evaluated with CT. Unsuspected retroperitoneal hematomas not requiring treatment have been reported in up to 13% of patients.30 Significant hemorrhage usually is caused by laceration of lobar arteries. Pseudoaneurysm, arteriovenous fistula, arteriocaliceal fistula, or frank extravasation may be seen at arteriography. The risk of hemorrhage is minimized by using fine needles for puncture, using appropriate guidance, and avoiding puncture of the anteromedial renal vessels. The vast majority of these complications can be managed endovascularly.31
Although there is always concern for infection in patients with the need for urinary decompression, sepsis only occurs in approximately 1% to 3.6% of patients undergoing nephrostomy placement (the higher incidence seen in patients undergoing decompression for suspected infection).32 Patients with infected urine or stones are at higher risk for PCN-related sepsis. For elective PCN for stone disease, antibiotic therapy ideally should be initiated and continued until the urine is clear. Diagnostic nephrostograms, ureteral catheterization, and stent placement should be delayed in patients with pyonephrosis until the urine has cleared.
Pleural complications include pneumothorax and empyema from infected urine entering the pleural space along the nephrostomy track. These complications are prevented by obtaining access beneath the 12th rib. One study showed that PCN tracks above the 12th rib traverse the pleura in 29% of cases on the right and 14% of cases on the left.33 Pleural complications occur in only about 0.2% of decompressive nephrostomies.21 When supracostal access is required for stone therapy, the risk of pleural complications increases to as much as 12%.34,35 However, many pleural transgressions remain clinically silent.
Minor perforations of the collecting system occur in about 2% of patients. If a satisfactory drainage catheter is placed in the collecting system, these leaks usually heal spontaneously over the next 48 to 72 hours. The risk of urine leak as well as hemorrhage is increased with a direct renal pelvis puncture (Fig. 23-12). Urinomas requiring drainage are less common and occur rarely with standard 8- to 12-Fr PCN tubes. After removal of larger PCN catheters used for nephrostolithotomy, urinomas are more common. Other rare complications of PCN include air embolism and puncture of the colon, spleen, liver, and gallbladder.36–39
Catheter dislodgment is a relatively common occurrence. In a recent review of 283 patients with 325 nephrostomy tubes, there was an episode of complete dislodgment of the catheter in 16.6% of the patients. The vast majority of the tubes were replaced through the preexisting track, with a limiting factor of track maturity. Successful replacement rates were 17%, 35%, and 97% in tracks less than 4 weeks old, less than 6 weeks old, and more than 6 weeks old, respectively.40 Some patients have recurrent dislodgment of their tubes. One author describes a solution by placement of a renal “U-tube.” This tube goes in one polar calyx and out the other pole with side holes within the collecting system.41 Malecot catheters commonly used after nephrostomy track dilation may become difficult to extract if tissue bridges grow over the catheter flanges.42 These devices should therefore be used with caution for long-term drainage. The entrapped catheters may be safely removed using endoscopic techniques to cut the anchoring tissue bridges.
Ureteral stent placement
Patient selection
Ureteral stent placement is indicated for a broad variety of clinical problems (Box 23-2). PCN tube placement with antegrade stent placement usually is reserved for patients in whom retrograde attempts are unsuccessful or not feasible. For patients with nephroureteral obstruction, ureteral stents have distinct advantages over external drainage through a PCN tube. The drainage with stents is internalized, eliminating the need for external drainage tubing and collecting bags. The completely internalized ureteral stent is tolerated better by patients, and it decreases the risk of urosepsis from the inevitable colonization of external drainage catheters.43,44 Ureteral stents also have several disadvantages. Stent malfunction may not be detected quickly and often requires sonography or cystography for diagnosis. Stent patency varies with the clinical situation. Stone-forming patients tend to develop rapid stent occlusion, and stent exchange may be required as frequently as every 6 weeks. In general, stents should be exchanged at least every 6 months with transurethral cystoscopic or fluoroscopic guidance to prevent encrustation and occlusion. Stents may cause debilitating irritation of the bladder wall or trigone. Some patients prefer externally draining PCN tubes because function can be assessed easily and replacement is simple.
External PCN drainage is preferred over ureteral stents in several situations, particularly when there are contraindications to ureteral stent placement (Box 23-3). In the occasional patient with vesicocutaneous fistula due to advanced pelvic malignancy, diversion of urine with ureteral occlusion is beneficial to facilitate fistula closure and minimize tissue breakdown and nursing care requirements. Nephroureteral catheters are available with ureteral occlusion balloons to treat this difficult group of patients.
Technique
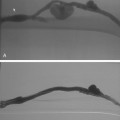
Almost all ureteral strictures can be traversed with a curved catheter and an angled hydrophilic guidewire (Fig. 23-13). If the catheter buckles in the renal pelvis, a sheath may be advanced into the proximal ureter. When the guidewire has crossed the obstruction, a catheter should be advanced and injected to confirm its location in the bladder. Vigorous or overzealous manipulation of the hydrophilic guidewire may easily puncture the urothelium. A 4-5 Fr hydrophilic catheter usually follows the hydrophilic guidewire into the urinary bladder. The hydrophilic guidewire should then be exchanged for a stiff guidewire. If necessary, the stricture is dilated to allow the ureteral stent to pass freely to the bladder. Dilators may be used to dilate to 1 Fr size larger than the ureteral stent. Alternatively, a 3- to 4-mm angioplasty balloon may be used.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree